Abstract
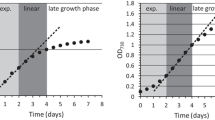
In batch cultures ofPseudomonas aeruginosa, hydrogen cyanide is produced primarily during the transition between logarithmic and stationary phases. This transient response is due to the synthesis of the enzyme system of cyanogenesis during mid to late logorithmic and the inactivation of this system in early stationary phase. Although glycine, the metabolic precursor of cyanide, stimulates cyanogenesis, it is not necessary to incorporate this amino acid in the growth medium to produce elevated enzyme levels. Under conditions of iron limitation (1×10−6 M), phosphate limitation (0.1 mM), and excess phosphate (250 mM), the culture produces low levels of the cyanogenic enzyme system. Increasing the carbon and energy source,l-glutamate, prolongs cyanogenesis and postpones the inactivation of the cyanogenic enzyme system.
Similar content being viewed by others
Literature Cited
Asmus, E., Garschagen, H. 1953. The use of barbituric acid for the photometric determination of cyanide and thiocyanate. Zeitschrift für Analytische Chemie138:414–422.
Castric, P. A. 1975. Hydrogen cyanide, a secondary metabolite ofPseudomonas aeruginosa. Canadian Journal of Microbiology21:613–618.
Castric, P. A. 1977. Glycine metabolism byPseudomonas aeruginosa: Hydrogen cyanide biosynthesis. Journal of Bacteriology130:826–831.
Demain, A. L., Piret, J. M., Friebel, T. E., Vandamme, E. J., Matteo, C. C. 1976. Studies onBacillus brevis directed towards the cell-free synthesis of gramicidin S, pp. 437–443. In: Schlessinger, D. (ed.), Microbiology—1976. Washington, D.C.: American Society for Microbiology.
Drew, S. W., Demain, A. L. 1977. Effect of primary metabolites on secondary metabolism. Annual Review of Microbiology31:343–356.
Fujikawa, K., Suzuki, T., Kurahashi, K. 1968. Biosynthesis of tyrocidine by a cell-free enzyme system ofBacillus brevis ATCC 8185. 1. Preparation of partially purified enzyme system and its properties. Biochimica et Biophysica Acta161:232–246.
Gallo, M., Katz, E. 1972. Regulation of secondary metabolite biosynthesis: Catabolite repression of phenoxazinone synthase and and actinomycin formation by glucose. Journal of Bacteriology109:659–667.
Knowles, C. J. 1976. Microorganisms and cyanide. Bacteriological Reviews40:652–680.
Krupinski, V. M., Robbers, J. E., Floss, H. G. 1976. Physiological study of ergot: Induction of alkaloid synthesis by tryptophan at the enzymatic level. Journal of Bacteriology125:158–165.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry193:265–275.
Matteo, C. C., Glade, M., Tanaka, A., Piret, J., Demain, A. L. 1975. Microbiological studies on the formation of gramicidin S synthetases. Biotechnology and Bioengineering17:129–142.
Meganathan, R., Castric, P. A. 1977. The effect of inorganic phosphate on cyanogenesis byPseudomonas aeruginosa. Archives of Microbiology114:51–54.
Niven, D. F., Collins, P. A., Knowles, C. J. 1975. The respiratory system ofChromobacterium violaceum grown under conditions of high and low cyanide evolution. Journal of General Microbiology90:271–285.
Rodgers, P. B., Knowles, C. J. 1978. Cyanide production and degradation during growth ofChromobacterium violaceum. Journal of General Microbiology108:261–267.
Switzer, R. L. 1977. The inactivation of enzymes in vivo. Annual Review of Microbiology31:135–157.
Weinberg, E. D. 1971. Secondary metabolism; Raison d’etre. Perspectives in Biology and Medicine14:565–577.
Weinberg, E. D. 1974. Secondary metabolism: Control by temperature and inorganic phosphate. Developments in Industrial Microbiology15:70–81.
Weinberg, E. D. 1977. Mineral element control of microbial secondary metabolism, pp. 289–315. In: Weinberg, E. D. (ed.), Microorganisms and minerals. New York: M. Dekker.
Wissing, F. 1968. Growth curves and pH-optima for cyanide producing bacteria. Physiologia Plantarum21:589–593.
Wissing, F. 1974. Cyanide formation from oxidation of glycine by aPseudomonas species. Journal of Bacteriology117:1289–1294.
Wissing, F. 1975. Cyanide production from glycine by a homogenate from aPseudomonas species. Journal of Bacteriology121:695–699.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Castric, P.A., Ebert, R.F. & Castric, K.F. The relationship between growth phase and cyanogenesis inPseudomonas aeruginosa . Current Microbiology 2, 287–292 (1979). https://doi.org/10.1007/BF02602861
Issue Date:
DOI: https://doi.org/10.1007/BF02602861