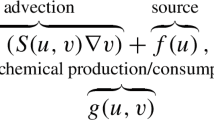Abstract
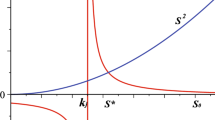
Oscillations in cytosolic Ca2+ concentrations in living cells are often a manifestation of propagating waves of Ca2+. Numerical simulations with a realistic model of inositol 1, 4, 5-trisphosphate (IP3)-induced Ca2+ wave trains lead to wave speeds that increase linearly at long times when (a) IP3 levels are in the range for Ca2+ oscillations, (b) a gradient of phase is established by either an initial ramp or pulse of IP3, and (c) IP3 concentrations asymptotically become uniform. We explore this phenomenon with analytical and numerical methods using a simple two-variable reduction of the De Young-Keizer model of the IP3 receptor that includes the influence of Ca2+ buffers. For concentrations of IP3 in the oscillatory regime, numerical solution of the resulting reaction diffusion equations produces nonlinear wave trains that shows the same asymptotic growth of wave speed. Due to buffering, diffusion of Ca2+ is quite slow and, as previously noted, these waves occur without appreciable bulk movement of Ca2+. Thus, following Neu and Murray, we explore the behavior of these waves using an asymptotic expansion based on the small size of the buffered diffusion constant for Ca2+. We find that the gradient in phase of the wave obeys Burgers' equation asymptotically in time. This result is used to explain the linear increase of the wave speed observed in the simulations.
Similar content being viewed by others
References
Allbritton, N. L., Meyer, T. and Stryer, L. 1992. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate.Science 258, 1812–1815.
Atri, A., Amundson, J., Clapham, D. and Sneyd, J. 1993. A single-pool model for intracellular calcium oscillations and waves in theXenopus-laevis oocyte.Biophys. J. 65, 1727–1739.
Berridge, M. J. 1993. Inositol trisphosphate and calcium signalling.Nature 361, 315–325.
Burgers, J. M. 1940. Application of a model system to illustrate some points of the statistical theory of free turbulence.Proc. Acad. Sci. Amst. 43, 2–12.
De Young, G. and Keizer, J. 1992. A single-pool isositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration.Proc. Natl. Acad. Sci. USA 89, 9895–9899.
Doedel, E. J., 1981. AUTO: a program for the automatic bifurcation analysis of autonomous systems.Cong. Num. 30, 265–284.
Dupont, G. and Goldbeter, A. 1994. Propertics of intracellular Ca2+ waves generated by a model based on Ca2+-induced Ca2+ release.Biophys. J. 67, 2191–2204.
Ermentrout, B. 1996. XPPAUTO: the differential equation solving tool. ftp://ftp.math.pitt.edu/pub/bardware/tut/start.html
Girard, S., Luckhoff, A., Lechleiter, J., Sneyd, J. and Clapham, D. 1992. Two-dimensional model of calcium waves reproduces the patterns observed inXenopus oocytes.Biophys. J. 61, 509–517.
Jafri, M. S. 1995. A theoretical study of cytosolic calcium waves.J. Theor. Biol. 172, 209–216.
Jafri, M. S. and Keizer, J. 1994. Diffusion of inositol 1,4,5-trisphosphate but not Ca2+ is necessary for a class of inositol 1,4,5-trisphosphate-induced Ca2+ waves.Proc. Natl. Acad. Sci. USA 91, 9485–9489.
Keizer, J. and De Young, G. 1994. Simplification of a realistic model of [IP3]-induced Ca oscillations.J. Theor. Biol. 166, 431–442.
Keizer, J., Li, Y.-X., Stojilković, S. and Rinzel, J. 1995. InsP3-induced Ca2+ excitability of the endoplasmic reticulum.Mol. Biol. Cell 6, 945–951.
Li, Y-X., Keizer, J., Stojilković, S. and Rinzel, J. 1995. Ca2+ excitability of the ER membrane: an explanation for IP3-induced Ca2+ oscillations.Am. J. Physiol. 269 (Cell Physiol. 28), C1079-C1092.
Li, Y. X. and Rinzel, J. 1994. Equations for insp(3) receptor-mediated [Ca2+](i) oscillations derived from a detailed kinetic model—a Hodgkin-Huxley like formalism.J. Theor. Biol. 166, 461–473.
Murray, J. D. 1990.Mathematical Biology. New York: Springer-Verlag.
Neu, J. C. 1979. Chemical waves and the diffusive coupling of limit cycle oscillators.SIAM J. Applied Math. 36, 509–515.
Wagner, J. and Keizer, J. 1994. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations.Biophys. J. 67, 447–456.
Watras, J., Brezprovanny, I. and Ehrlich, B. 1991. Inositol 1,4,5 trisphosphate-gated channels in cerebellum: presence of multiple conductance states.J. Neurosci. 11, 3239–3245.
Whitham, G. B. 1974.Linear and Nonlinear Waves, 1st ed. New York: Wiley.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jafri, M.S., Keizer, J. Agonist-induced calcium waves in oscillatory cells: A biological example of Burgers' equation. Bltn Mathcal Biology 59, 1125–1144 (1997). https://doi.org/10.1007/BF02460104
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02460104