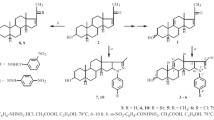
Abstract
Data on the synthesis and biological activity of isoxazole derivatives of steroids have been correlated.
Similar content being viewed by others
References
A. A. Akhrem and Yu. A. Titov,Usp. Khim.,36, 745 (1967).
A. V. Kamernitzky and A. M. Turuta,Heterocycles,7, 547 (1977).
C. Camoutsis,J. Heterocycl. Chem.,33, 539 (1996).
A. J. Manson, F. W. Stonner, H. C. Neumann, R. G. Christiansen, R. L. Clarke, J. H. Ackerman, D. F. Page, J. W. Dean, D. K. Phillips, G. O. Potts, A. Arnold, A. L. Beyler, and R. O. Clinton,J. Med. Chem.,6, 1 (1963).
E. Caspi and D. M. Piatak,Can J. Chem.,41, 2294 (1963).
R. O. Clinton and A. J. Manson, US Pat. 3135743;Chem. Abstr.,61, 5730 (1964).
P. J. Palmer,J. Chem. Soc., No. 7, 3901 (1963).
J. C. Babcock and J. A. Campbell, US Pat. 3980638;Chem. Abstr.,86, 43912 (1977).
F. Winternitz, C. Menou, and E. Arnal,Bull. Soc. Chim. France, 505 (1960).
R. G. Christiansen, Ger. Pat. 2855091;Chem. Abs.,92, 6812 (1980).
E. Caspi and D. M. Piatak,Chem. Ind., 1984 (1962).
R. O. Clinton, US Pat. 3515735;Chem. Abstr.,73, 45694 (1970).
T. Nambara, K. Shimada, S. Iwamura, M. Mori, and M. Nokubo,Chem. Pharm. Bull. 21, 2474 (1973).
A. A. Shishkina, T. I. Ivanenko, V. M. Rzheznikov, and K. K. Pivnitskii,Khim.-farm. Zh.,8, No. 1, 7 (1976).
P. Ruggieri, C. Gandolfi, and U. Guzzi,Tetrahedron Lett., No. 50, 4603 (1965).
V. Kumar and A. B. Todaro,Synthesis, No. 6, 523 (1992).
S. Giacopello, M. E. Deluca, and A. M. Seldes,Z. Naturforsch. B,47, 891 (1992).
E. Marchetti and P. Donini,Gazz. Chim. Ital.,91, 1133 (1961).
P. J. Dharam and R. Y. Mange, Indian Pat. 166480;Chem. Abstr.,117, 49006 (1992).
M. A. Viswamitra, R. Radhakrishnan, J. Bandekar, and G. R. Desiraju,J. Am. Chem. Soc.,115, 4868 (1993).
L. H. Briggs, J. P. Bartley, and P. S. Rutledge,J. Chem. Soc. C, No. 11, 2115 (1971).
R. W. Guthrie, R. W. Kierstead, and R. A. Le Mahieu, US Pat. 3869467;Chem. Abstr.,83, 43609 (1975).
P. Ruggieri, C. Gandolfi, and U. Guzzi,Tetrahedron Lett., No. 2, 205 (1966).
R. G. Christiansen and J. C. Collins, Ger. Pat. 3018903;Chem. Abstr.,95, 25400 (1981).
H. Kaneco, K. Nakamura, J. Jamato, and M. Kurokawa,Chem. Pharm. Bull.,17, 11 (1969).
K. Bruckner, K. Irmsher, F. Werder, K.-H. Bork, and H. Mets,Chem. Ber.,94, 2897 (1961).
D. Bertin and L. Nedelec, US Pat. 3629245;Chem. Abstr.,76, 86006 (1972).
Y. Ling, I. Li, Y. Liu, K. Kato, G. T. Klus, and A. Brodie,J. Med. Chem.,40, 3297 (1997).
A. U. Siddiqui, A. H. Siddiqui, and T. S. Ramian,J. Indian Chem. Soc.,69, 282 (1992).
W. Fritsch, G. Seidl, and H. Ruschig,Liebigs Ann. Chem. 677, 139 (1964).
U. Stache, W. Fritsch, and H. Ruschig,Liebigs Ann. Chem.,685, 228 (1965).
G. W. Moersch, E. L. Wittle, and W. A. Neuklis,J. Org. Chem.,40, 1272 (1965).
T. Kwon, A. S. Heiman, E. T. Oriaku, K. Yoon, and H. J. Lee,J. Med. Chem.,38, 1048 (1995).
M. Khalil, M. F. Maponya, D.-H. Ko, Z. You, E. T. Oriaku, and H. J. Lee,Med. Chem. Res., No. 6, 52 (1996).
A. Ius, C. Parini, G. Sportoletti, G. Vecchio, and G. Ferrara,J. Org. Chem.,36, 3470 (1971).
T. Kametani, H. Furuyuma, and T. Honda,Heterocycles,19, 357 (1982).
I. S. Levina, E. I. Mortikova, and A. V. Kamernitzky,Synthesis, No. 8, 562 (1974).
A. V. Kamernitzky and I. S. Levina,Izv. Akad. Nauk SSSR, Ser. Khim. No. 5, 1139 (1980).
A. V. Kamernitzky, I. S. Levina, and E. M. Mortikova,Izv. Akad. Nauk SSSR, Ser. Khim., No. 8, 1924 (1977).
A. V. Kamernitzky, I. S. Levina, E. I. Mortikova, and B. S. El'yanov,Tetrahedron Lett., No. 37, 3235 (1975).
I. S. Levina, A. V. Kamernitzky, E. I. Mortikova, B. S. El'yanov, and V. M. Zhulin, Authors Certificate USSR 491625;Byull. Izobret., No. 42, 60 (1975).
C. Parini, S. Colombi, A. Iric, R. Longhi, and G. Vecchio,Gazz. Chim. Ital.,107, 559 (1977).
J. Kalvoda and H. Kaufmann,J. Chem. Soc., Chem. Commun., No. 6, 209 (1976).
H. Kaufman and J. Kalvoda,J. Chem. Soc., Chem. Commun., No. 6, 210 (1976).
H. Laurent,Rev. Soc. Quim. Mex.,13, 218A, (1969).
A. A. Akhrem, F. A. Lakhvich, and V. A. Khripach,Zh. Obshch. Khim.,45, 2572 (1975).
A. V. Baranovsky, R. P. Litvinovskaya, and V. A. Khripach,Zh. Org. Khim.,26, 1274 (1990).
N. I. Garbuz, G. P. Yankovskaya, A. V. Baranovsky, R. P. Litvinovskaya, and V. A. Khripach,Khim. Prir. Soedin., No. 3, 391 (1994).
A. A. Akhrem, V. A. Khripach, F. A. Lakhvich, M. I. Zavadskaya, O. A. Drachenova, and I. A. Zorina,Dokl. Akad. Nauk SSSR,297, 364 (1987).
A. A. Akhrem, V. A. Khripach, F. A. Lakhvich, M. I. Zavadskaya, O. A. Drachenova, and I. A. Zorina,Zh. Org. Khim.,25, 2120 (1989).
A. I. Verenich, A. A. Govorova, N. M. Galitskii, A. V. Baranovskii, R. P. Litvinovskaya, and V. A. Khripach,Zh. Struk. Khim., No. 5, 152 (1992).
R. K. Tkhaper, I. G. Reshetova, and A. V. Kamernitzky,Izv. Akad. Nauk SSSR, Ser. Khim., No. 11, 2646 (1991).
R. K. Tkhaper, I. G. Reshetova, A. V. Kamernitzky, and R. P. Litvinovskaya,Izv. Akad. Nauk SSSR, Ser. Khim., No. 4, 969 (1991).
A. A. Akhrem, V. A. Khripach, R. P. Litvinovskaya, A. V. Baranovsky, M. I. Zavadskaya, A. N. Kharitonovich, E. V. Borisov, and F. A. Lakhvich,Zh. Org. Khim.,25, 1901 (1989).
V. A. Khripach, R. P. Litvinovskaya, A. V. Baranovsky, and A. A. Akhrem,Dokl. Akad. Nauk SSSR,318, 597 (1991).
V. Khripach, R. Litvinovskaya, A. Baranovsky, and S. Drach,Tetrahedron Lett.,31, 7065 (1990).
V. Khripach, V. Zhabinskii, and R. Litvinovskaya, in:Brassinosteroids: Chemistry, Bioactivity and Application, H. G. Gulter, T. Yokota, and G. Adam (editors), Am. Chem. Soc., Washington (1991), p. 43.
V. A. Khripach, R. P. Litvinovskaya, and S. V. Drach,Zh. Org. Khim.,29, 717 (1993).
R. P. Litvinovskaya, S. V. Drach, and V. A. Khripach,Mendeleev Commun., No. 6, 215 (1995)
R. P. Litvinovskaya, V. A. Tereshko, S. V. Drach, and V. A. Khripach,Zh. Obshch. Khim.,66, 859 (1996).
R. P. Litvinovskaya, A. S. Lyakhov, A. A. Gororova, S. V. Drach, and V. A. Khripach,Bioorg. Khim.,23, 147 (1997).
V. A. Khripach, R. P. Litvinovskaya, S. V. Drach, and E. A. Ermolenko,Zh. Org. Khim.,34, 1037 (1998).
V. A. Khripach, V. N. Zhabinskii, V. K. Ol'khovik, M. I. Zavadskaya, O. A. Drachenova, and N. B. Khripach,Zh. Org. Khim.,32, 841 (1996).
R. P. Litvinovskaya, H. V. Koval', and V. A. Khripach,Khim. Geterotsikl. Soedin., No. 2, 267 (1998).
V. A. Khripach, V. N. Zhabinskii, and A. I. Kotyatkina,Zh. Org. Khim.,29, 1569 (1993).
V. A. Khripach, V. N. Zhabinskii, and A. I. Kotyatkina,Zh. Org. Khim.,29, 1573 (1993).
R. P. Litvinovskaya, N. V. Koval', and V. A. Khripach,Zh. Org. Khim. 33, 1522 (1997).
R. P. Litvinovskaya, S. V. Drach, and V. A. Khripach,Zh. Org. Khim. 33, 201 (1997).
V. A. Khripach, V. N. Zhabinskii, and N. D. Pavlovskii,Zh. Org. Khim.,34, 59 (1998).
J. M. Midgley, J. E. Parkin, and W. B. Whalle,J. Chem. Soc. D. No. 13, 789 (1970).
L. A. Maldonado and P. Crabbe,Chem. Ind. No. 35, 1146 (1970).
P. Crabbe, L. A. Maldonado, and I. Sanchez,Tetrahedron,27, 711 (1971).
L. I. Klimova and N. N. Suvorov,Zh. Obshch. Khim.,34, 1357 (1964).
S. Noguchi, M. Imanishi, and K. Morita,Chem. Pharm. Bull.,12, 1189 (1964).
Roussel-UCLAF, Fr. Pat. 1780;Chem. Abstr.,59, 14061 (1963).
Roussel-UCLAF, Belg. Pat. 624199;Chem. Abstr.,60, 12083 (1964).
A. U. Siddiqui, D. Ramesh, Y. Satyanarayana, M. Srinivas, and A. H. Siddiqui,Org. Prep. Proced. Int.,25, 356 (1993).
A. H. Siddiqui, Y. Satyanarayana, M. Srinivas, and K. Rajeshwar,J. Indian Chem. Soc.,69, 846 (1992).
L. N. Volovel'skii, N. V. Popova, and I. P. Fal'ko, Authors Certificate USSR 447402;Byull. Izobret., No. 39 (1974).
L. N. Volovel'skii, N. V. Popova, M. Ya. Yakovleva, and V. G. Khukhryanskii,Zh. Obshch. Khim.,45, 2090 (1975).
L. N. Volovel'skii, I. B. Skachek, and I. I. Kuz'menko,Khim. Prir. Soedin. No. 2, 168 (1979).
J. R. Housley, Brit. Pat. 1287271;Chem. Abstr.,77, 152462 (1972).
G. S. Khatamkulova, M. I. Goryaev, and M. P. Irismetov,Izv. Akad. Nauk KazSSR, Ser. Khim., No. 4, 71 (1974).
M. P. Irismetov and M. I. Goryaev,Trud. Inst. Khim. Nauk, Akad. Nauk KazSSR 52, 17 (1980).
M. P. Irismetov, M. I. Goryaev, and G. Yu. Tsvetkova,Zh. Obshch. Khim.,46, 1407 (1976).
H. Chanh and J. Sylvestre,Bull. Soc. Chim. France, No. 10, 3586 (1971).
D. Burn, G. Cooley, J. W. Ducler, B. Ellis, D. M. Kirk, and V. Petrow,Tetrahedron Lett., No. 13–14, 733 (1964).
Y. Sato and H. Kaneko,Steroids,5, 279 (1965).
J. T. Pinhey, E. Rizzardo, and G. C. Smith,Aust. J. Chem.,31, 113 (1978).
J. T. Pinhey, E. Rizzardo, and G. C. Smith,Aust. J. Chem.,31, 97 (1978).
H. Suginome, K. Macoto, O. Toshiharu, J. Shinji, O. Takashi, S. Hisanori, and F. Akio,J. Chem. Soc. Perkin Trans. 1, No. 4, 427 (1992).
J. Boivin, S. Huppe, and S. Z. Zard,Tetrahedron Lett.,37, 8735 (1996).
R. G. Christiansen, US Pat. 4055562;Chem. Abstr.,88, 121545 (1978).
V. Kumar and M. R. Bell,Heterocycles,29, 1773 (1989).
H. C. Neumann, G. O. Potts, W. T. Ryan, and F. W. Stonner,J. Med. Chem.,13, 948 (1970).
R. P. Litvinovskaya, A. V. Baranovsky, and V. A. Khripach,Zh. Org. Khim.,33, 1350 (1997).
R. G. Christiansen, Can. Pat. 1125278;Chem. Abstr.,97, 216572 (1982).
R. G. Christiansen, US Pat. 4160027;Chem. Abstr.,91, 211680 (1979).
K. O. Gelotte and J. Chester, US Pat. 4755595;Chem. Abstr.,110, 115182 (1989).
W. E. Jones and R. S. Andrews, Eur. Pat. 154476;Chem. Abstr.,105, 60813 (1986).
R. O. Clinton and A. J. Manson, Brit. Pat. 1123770;Chem. Abstr.,85, 108895 (1976).
G. Nathansohn, G. Winters, and E. Testa, US Pat. 3459740;Chem. Abstr.,71, 102135 (1969).
T. Tamaya, N. Furuta, T. Motoyama, S. Boku, Y. Ohono, and H. Okada,Acta Endocrinol.,88, 190 (1978).
J. F. Shi, Q. J. Liao, and F. Xu,Yaoxue Xuebao,23, 860 (1988).
R. P. Litvinovskaya, S. V. Drach, A. V. Baranovsky, V. A. Strel'tsova, and V. A. Khripach,Vestsi Akad. Navuk Belarus, Ser. Biyal. Navuk, No. 2, 49 (1996).
V. A. Khripach, R. P. Litvinovskaya, S. V. Drach, and V. A. Strel'tsova, Authors Certificate USSR 1786807;Byull. Izobret., No. 3, 321 (1996).
V. A. Khripach, R. P. Litvinovskaya, S. V. Drach, and V. A. Strel'tsova, Authors Certificate USSR 174755;Byull. Izobret., No. 26, 72 (1992).
Additional information
Institute of Bioorganic Chemistry, National Academy of Sciences of Belarus, Minsk 220141; Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 291–314, March, 2000.
Rights and permissions
About this article
Cite this article
Drach, S.V., Litvinovskaya, R.P. & Khripach, V.A. Steroidal 1,2-oxazoles. Synthesis and biological activity. (Review). Chem Heterocycl Compd 36, 233–255 (2000). https://doi.org/10.1007/BF02256860
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02256860