Abstract
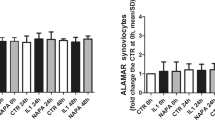
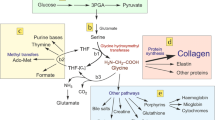
Cartilage destruction is a characteristic feature of osteoarthritis. Treatment with certain nonsteroidal anti-inflammatory drugs could exacerbate cartilage destruction by impairing the synthesis of cartilage matrix proteins, type II collagen and proteoglycan. In order to monitor the changes occurring in cartilage collagen synthesis, we developed a type II collagen specific ELISA. The effects of antiarthritic agents on type II collagen and glycosaminoglycan synthesis were examined in rat chondrosarcoma cultures. Drugs were added to the monolayer cultures and 4 days later the total type II collagen, as determined by the type II collagen ELISA, and glycosaminoglycan content, as measured by dimethylmethylene blue dye binding assay, was measured. All drugs except tiaprofenic acid decreased type II collagen synthesis by at least 40% at 100 μg/ml. Tiaprofenic acid at 1 μg/ml increased type II collagen content by 54% of the controls. Glycosaminoglycan synthesis was decreased by acetylsalicylic acid, diclofenac and tiaprofenac acid, at 50 μg/ml or above. Indomethacin, naproxen and dexamethasone had no effect. Interestingly, tenidap stimulated the glycoaminoglycan synthesis by 32% at 100 μg/ml. We show that the combination of chondrosarcoma cultures, type II collagen specific ELISA and dimethylmethylene blue dye binding assay serves as a useful model for screening the effects of agents capable of modulating type II collagen and glycosaminoglycan synthesis.
Similar content being viewed by others
References
D. Hammerman,The biology of osteoarthritis. New. Engl. J. Med.320, 1322–1331 (1989).
D. R. Eyre and J. J. Wu,Collagen of fibrocartilage: A distinctive molecular phenotype in bovine meniscus. FEBS Lett.158, 265–273 (1983).
R. Mayne,Cartilage collagens: What is their function and are they involved in articular disease? Arth. Rheum. 32, 241–246 (1989).
G. R. Srinivas, H.-J. Barrach and C. O. Chichester,Quantitative immunoassays to type II collagen and its cyanogen bromide peptides. J. Immunol. Meth.159, 53–62 (1993).
B. D. Smith, G. R. Martin, E. J. Miller, A. Dorfman and R. Swarm,The nature of the collagen synthesized by a transplanted chondrosarcoma. Arch. Biochem. Biophys.166, 181–186 (1975).
J. H. Kimura, L. S. Lohmander and V. C. Hascall,Studies on the biosynthesis of cartilage proteoglycan in a model system of cultured chondrocytes from Swarm rat chondrosarcoma. J. Cell. Biochem.26, 261–278 (1984).
S. Collier and P. Ghosh,Comparison of the effects of non-steroidal antiinflammatory drugs (NSAIDs) on proteoglycan synthesis by articular cartilage explant and chondrocyte monolayer cultures. Biochem. Pharmacol.41, 1375–1384 (1991).
H. Muir, S. L. Carney and L. G. Hall,Effects of tiaprofenic acid and other NSAIDs on proteoglycan metabolism in articular cartilage explants. Drugs25 (Suppl. 1) 15–23 (1988).
C. T. Bassleer, Y. E. Henrotin, J.-Y. L. Reginster and P. P. Franchimont,Effects of tiaprofenic acid and acetylsalicylic acid on human articular chondrocytes in 3-dimensional culture. J. Rheumatol.19, 1433–1438 (1992).
B. J. deVires, W. B. van den Berg, E. Vitters and B. A. Levinus van de Putte,Effects of NSAIDs on the metabolism of sulphated glycosaminoglycans in healthy and (post) arthritic murine articular cartilage. Drugs35, 24–32 (1988).
K. Fujii, K. Tajiri, S. Sai, T. Tanaka and K. Murota,Effects of nonsteroidal antiinflammatory drugs on collagen biosynthesis of cultured chondrocytes. Sem. Arth. Rheum.18, 16–18 (1989).
A. Mauviel, F. Redini, G. Loyau and J.-P. Pujol,Modulation of extracellular matrix metabolism in rabbit articular chondrocytes and human rheumatoid synovial cells by nonsteroidal antiinflammatory drug etodolac I: Collagen synthesis. Agents and Actions31, 345–352 (1990).
T. R. Oegema, V. C. Hascall, D. D. Dziewiatowski,Isolation and characterization of proteoglycans from the Swarm rat chondrosarcoma. J. Biol. Chem.250, 6151–6159 (1975).
R. W. Farnsdale, C. A. Sayers and A. J. Barret,A direct spectrophotometric microassay for sulfated glycoaminoglycans in cartilage cultures. Connective Tissue Res.9, 247–248 (1982).
R. T. Hinegardner,An improved fluorometric assay for DNA. Anal. Biochem.39, 197–201 (1971).
Y. Henrotin, C. Bassleer and P. Franchimont,In vitro effects of etodolac and acetylsalicylic acid on human chondrocyte metabolism. Agents and Actions36, 317–323 (1992).
P. Netter, B. Barnwarth and M.-J. Royer-Morrot,Recent finding of non-steroidal antiinflammatory drugs in synovial fluid. Clin. Pharmacokinet.17, 145–162 (1989).
W. J. Wallis and P. A. Simkin,Antirheumatic drug concentrations in human synovial fluid and synovial tissue. Observations on extravascular pharmacokinetics. Clin. Pharmacokinet.8, 496–522 (1983).
M. Franke, G. Manz and J. P. Glynn,Distribution of benorylate in plasma, synovial fluid and tissue in rheumatoid arthritis. Scand. J. Rheumatol.13, 13–17 (1976).
H. Spahn, K. Thabe, E. Mutscheller, K. Tillmann and I. Giklor,Concentration of azapropazone in synovial tissue and fluid, Eur. J. Pharmacol.32, 303–307 (1987).
S. Jalava, H. Saarimaa, M. Anttila and H. Sundquist,Naproxen concentrations in serum, synovial fluid and synovium. Scand. J. Rheumatol.6, 155–157 (1977).
M. Farr,Investigation of phenylbutazone in synovial fluid. J. Int. Med. Res.5, 26–29 (1977).
A. Gaucher, P. Netter, G. Faure, J. P. Schoeller and A. Gerarlin,Diffusion of oxyphenylbutazone into synovial fluid, synovial tissue, joint cartilage and cerebrospinal fluid. Eur. J. Clin. Pharmacol.25, 107–112 (1983).
R. E. Peterson, R. L. Black and J. J. Bunim,Disposition of intraarticularly injected cortisone and hydrocortisone. Arth. Rheum.2, 433–439 (1959).
R. Luukkainen, M. Hakala, E. S. K. O. Sajanti, U. Hettuhtala, Yli-Kerttula and R. Hameenkorpi,Predictive value of synovial fluid analysis in estimating the efficacy of intraarticular corticosteroid injections in patients with rheumatoid arthritis. Ann. Rheum. Dis.51, 874–876 (1992).
A. I. Oikarinen, I. E. Vuorio, E. J. Zaragoza, A. Palotie, M. L. Chu and J. Uitto,Modulation of collagen metabolism by glucocorticoids. Receptor mediated effects of dexamethasone on collagen biosynthesis in chick embryo fibroblasts and chondrocytes. Biochem. Pharmacol.37, 1451–1462 (1988).
P. D. Benya and J. D. Shaffer,Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell30, 215–234 (1982).
P. D. Benya, S. R. Padilla and M. E. Nimni,The progeny of rabbit articular chondrocytes synthesize collagen types I and III and type I trimer, but not type II — verifications by cyanogen bromide peptide analysis. Biochemistry16, 865–872 (1977).
E. J. Miller and S. Gay,The collagens: An overview and up to date. InMethods of Enzymology, Vol. 144 (Eds S. P. Colowick and N. O. Kaplan) pp. 3–41, Academic Press, FL 1987.
M. B. Goldring, E. Sobbat, J. M. Elwell and J. Y. Chang,Etodolac preserves cartilage specific phenotype in human chondrocytes: Effects on type II collagen synthesis and associated mRNA levels. Eur. J. Rheumatol. Inflamm.10, 10–21 (1990).
M. Shinmei, T. Kikuchi, K. Matsuda and K. Shimomura,Effects of interleukin-1 and antiinflammatory drugs on the degradation of human articular cartilage. Drugs35, 33–41 (1988).
E. Vignon, P. Mathieu, P. Louisot, J. Vilamitjana, M. F. Harmand and M. Richard, Phospholipase A2 activity in human osteoarthritic cartilage. J. Rheumatol.16, 35–38 (1989).
J.-P. Pelletier, J.-M. Cloutier and J.-M. Pelletier,In vitro effects of tiaprofenic acid, sodium salicylate and hydrocortisone on the proteoglycan metabolism of human osteoarthritic cartilage. J. Rheumatol.16, 646–655 (1989).
F. Redini, A. Mauviel, G. Loyau and J.-P. Pujol,Modulation of extracellular matrix metabolism in rabbit articular chondrocytes and human rheumatoid synovial cells by the non steroidal anti-inflammatory drug etodolac II: Glycosaminoglycan synthesis. Agents and Actions31, 358–367 (1990).
M. K. Bansal, H. Ward and R. M. Mason,Proteoglycan synthesis in suspension cultures of Swarm rat chondrosarcoma chondrocytes and inhibition by exogeneous hyaluronate. Arch. Biochem. Biophys.246, 602–610 (1986).
C. J. Handley, P. Brooks and D. A. Lowther,Suppression of collagen synthesis by chondrocytes by exogenous concentrations of proteoglycan subunit. Biochem. Int.1, 270–276 (1980).
K. D. Gibson, B. J. Segen and T. K. Audhya,The effect of β-d-xylosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage in the absence of protein synthesis inhibitors. Biochem. J.162, 217–233 (1977).
A. Arufflo, I. Stamenkovic, M. Melnick, C. B. Underhill and B. Seed,CD44 is the principal cell surface receptor for hyaluronate. Cell61, 1303–1313 (1990).
M. J. Palmoski and K. D. Brandt,Effects of some nonsteroidal antiinflammatory drugs on proteoglycan metabolism and organization in canine articular cartilage. Arth. Rhem.23, 1010–1020 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Srinivas, G.R., Chichester, C.O., Barrach, H.J. et al. Effects of certain antiarthritic agents on the synthesis of type II collagen and glycosaminoglycans in rat chondrosarcoma cultures. Agents and Actions 41, 193–199 (1994). https://doi.org/10.1007/BF02001916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02001916