Summary
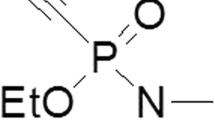
The reduction of acetylcholine esterase (AChE) activity or the complete blocking of AChE to be observed by histochemical demonstration of AChE in tissue after experimental and spontaneous (human) organophosphate intoxication (especially paraoxone = E 600 and parathion = E 605) should be interpreted as an indication of an in vivo inhibition of the cholinergic system. In animal experiments, a relationship was demonstrated between AChE activity and the applied dose of organophosphorous compounds. In addition, enzyme inhibition was observed in in vitro systems using AChE-containing mouse tissue sections pretreated with organophosphate solutions or with body fluids containing organophosphates. Examination of the concentration dependency indicated that the inhibiting solution must contain at least 0.15 μg/ml paraoxone or 5 mg/ml parathion to block AChE in the section. Using the same in vitro system, a half-life of 6–7 min was established for the paraoxone inactivating enzyme in blood. The in vivo and in vitro inhibited AChE was reactivated by consecutive treatment of blocked sections with toxogonin. This possibility of reactivation therefore allows qualitative classifications of the AChE-inhibiting toxin to the alkylphosphates. The postmortem persistence of the AChE inhibitory effect was demonstrable for about a 2-month interval. Since the histochemically demonstrable activity of the enzyme AChE is more or less constant during a postmortem interval of at least 70h, the model of histochemical demonstration is a method which provides a morphological equivalent for acute organophosphate intoxication.
Zusammenfassung
Die Reduktion oder vollständige Hemmung der histochemisch nachweisbaren Acetylcholinesterase (AChE)-Aktivität nach experimentellen und spontanen (menschlichen) Organophosphatvergiftungen (besonders mittels Paraoxon = E 600 und Parathion = E 605) muß als Zeichen einer in vivo-Hemmung des cholinergen Systems interpretiert werden. Im Tierversuch wurde eine Beziehung zwischen der AChE-Aktivität und applizierten Dosis der Organophosphate nachgewiesen. In einem in vitro-System konnte die Enzymhemmung nach Exposition von AChE-enthaltenden histologischen Schnitten mit Organophosphat-enthaltenden Körperflüssigkeiten bzw. angesetzten Lösungen festgestellt werden. Bei Untersuchung der Dosisabhängigkeit wurde ferner festgestellt, daß mindestens 0.15 μg/ml Paraoxon oder 5 mg/ml Parathion notwendig sind, um in vitro die AChE zu blockieren. Bei Anwendung desselben in vitro-Systems konnte eine Halbwertzeit für das Paraoxoninaktivierende Enzym im Blut von 6–7 min nachgewiesen werden. Die in vivo und in vitro gehemmte AChE war bei anschließender Behandlung der Schnitte mit Toxogonin reaktivierbar; die Möglichkeit der Reaktivation erlaubt eine qualitative Zuordnung des AChE-hemmenden Toxins zu den Alkylphosphaten. Eine postmortale Persistenz der Enzymhemmung war am menschlichen Material für ca. 2 Monate nachweisbar. Da auch die Acetylcholinesterase in weitgehend unveränderter Aktivität nach einem postmortalen Intervall von wenigstens 70 h nachweisbar ist, muß der histochemische Nachweis als morphologisches Äquivalent einer akuten Organophosphatintoxikation angesehen werden.
Similar content being viewed by others
References
Adebahr G (1960) Nierenveränderungen bei der E 605-Vergiftung des Menschen. Arch Toxikol 18:107–119
Aldridge WN (1953a) Serum esterases. 1. Two types of esterase (A and B) hydrolysing p-nitrophenylacetate, -propionate and butyrate, and a method for their determination. Biochem J 53:110–117
Aldridge WN (1953b) Serum esterases. 2. An enzyme hydrolysing diethyl-p-nitrophenylphosphate (E 600) and its identity with the A-esterase of mammalian sera. Biochem J 53: 117–124
Aldridge WN (1980) Acetylcholinesterase and other esterases inhibitors. In: Sandler M (ed) Enzyme inhibitors as drugs. Mac Millan, Hampshire, pp 115–125
Alsen C, Herrlinger A, Ohnesorge FK (1973) Eigenschaften der Cholinesterasen in Geweben des Dorsches (Gadus callarias) und ihre Inaktivierung durch in vivo Einwirkung von Paraoxon und Tabun. Arch Toxikol 30:263–275
Augustinsson KB (1948) Cholinesterase a study comparative enzymology. Acta Physiol Scand [Suppl 52] 15:1–181
Baker T, Glazner R, Lowndes HE (1977) Subacute neuropathic effects of diisoprophylfluorophosphate at the cat so leus neuromuscular junction. Neuropathol Appl Neurobiol 3:377–390
Barsotti M, Sassi C, Del Bono P (1954) Un caso di intossicazioni grave professionale da parathion con sindrome nefrosica. Med Lavoro 45:469–473
Bergner AD, Durlacher SH (1951) Histochemical detection of fatal anticholinesterase poisoning. Am J Pathol 27:1011–1021
Bergner DA, Bayliss MW (1952) Histochemical detection of fatal anticholinesterase poisoning. US Armed Forces Med J 3:1637–1644
Böhmer K (1955) Beitrag zur Kenntnis der Vergiftung durch E 605. Dtsch Z Gerichtl Med 44: 432–433
Bonhoff (1955) Diskussionsbemerkungen zum Vortrag von K Böhmer. Dtsch Z Gerichtl Med 44:433–434
Caruso A, Tessitore V (1968) Rilievi histochimici e considerazioni medico-legali sulle attivita colinesterasiche muscolari nell' avvelenamento acuto sperimentale da Rogor L. Zacchia 4: 335–346
Davison AN (1957) The conversion of schraden (OMPA) and parathion into inhibitors of cholinesterase by mammalian liver. Biochem J 61:302–303
Diggle WM, Gage JC (1951) Cholinesterase inhibition by parathion in vivo. Nature 168: 998
El-Badawi A, Schenk EA (1967) Histochemical methods for separate consecutive and simultaneous demonstration of acetylcholin-esterase and norepinephrine in cryostat sections. J Histochem Cytochem 15:580–588
Erdös EG, Boggs LE (1961) Hydrolysis of paraoxon in mammalian blood. Nature 190:716–717
Flügel M, Geldmacher-von Mallinckrodt M (1978) Zur Kinetik des Paraxon-spaltenden Enzyms im menschlichen Serum (EC 3.1.1.2). Klin Wochenschr 56:911–916
Frada G, Salamone L, Rizzo A (1964) Gli esteri fosforici. Patologia e clinica. Denaro, Palermo
Friedberg KD, Sakai F (1958) Spezifischer Nachweis von Vergiftungen mit Alkylphosphaten (E 600, E 605, Systox) in Blut und Hirngewebe mit Hilfe eines fermentreaktivierenden Antidots (Pyridin-Aldoxim-Methjodid, PAM). Dtsch Z Gerichtl Med 47:580–598
Geldmacher-von Mallinckrodt M (1975) Forensische Toxikologie. In: Mueller B (Hrsg) Gerichtliche Medizin. Springer, Berlin Heidelberg New York, S 691–988
Geldmacher-vonMallinckrodt M (1978) Polymorphism of human serum paraoxinase. Hum Genet [Suppl I]:65–68
Geldmacher-von Mallinckrodt M, Baumgartner W, Petenyi M, Burgis H, Lindorf HH, Metzner H (1972) Korrelation zwischen der unterschiedlichen Vergiftbarkeit der Serum-Cholinesterase durch E 600 und der Aktivität des E 600-spaltenden Enzymsystems in menschlichen Seren. Hoppe-Seyler's Z Physiol Chem 353:217–220
Geldmacher-von Mallinckrodt M, Schütz HW, Enders P (1978) Über den Abbau von Paraoxon im Serum nach der Blutentnahme. Vortrag 57. Kongreß der Deutschen Gesellschaft für Rechtsmedizin, Düsseldorf
Goedde HW, Doenicke A, Alstland K (1967) Pseudocholinesterasen. Springer, Berlin Heidelberg New York
Graev M, Fabroni F (1960) Dimostrazione istochimica della colinesterasi quale contributo alla diagnosi di avvelensmento da insetticidi organ-fosforici. Riv Med Leg Legisl Sanit 2:79–86
Gwyn DG, Heardman V (1965) A cholinesterase-Bielschowsky staining method for mammalian motor end plates. Stain Technol 40:15–18
Holmstedt B, Krook L, Rooney DR (1957) The pathology of experimental cholinesterase inhibitor poisoning. Acta Pharmacol Toxicol (Copenh) 13:337–344
Jääskeläinen AJ, Alba A (1969) Histochemically observable alterations in enzyme pattern of rat myocardium, caused by parathion (E 605). Acta Pharmacol Toxicol (Copenh) 27: 112–119
Karnovsky MJ, Roots L (1964) A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem 12:219–221
Klein H (1956) Die Speicheldrüse bei E605-Vergiftung. Dtsch Z Gerichl Med 45:510–515
Koelle GB (1963) Cholinesterase and anticholinesterase agents. In: Eichler O, Farah A (Hrsg) Handbuch der experimentellen Pharmakologie, Ergänzungswerk, Bd 15. Springer, Berlin Göttingen Heidelberg
Krisch K (1968) Enzymatische Hydrolyse von Diäthyl-p-nitrophenylphosphat (E600) durch menschliches Serum. Z Klin Chem Biochem 6:41–45
Kubistova J (1959) Parathion metabolism in female rat. Arch Int Pharmacodyn 118:308–316
Maayani S, Weinstein H (1979) Some structure activity relationship of phencyclidine derivatives as anticholinergic agents in vitro and in vivo. In: Sharp CG, Abood LG (eds) Membrane mechanisms of drugs of abuse. Liss, New York, pp 91–106
Mancha LS De la, Verge DE, Bouchaud C, Coq H, Sentenac-Roumanou H (1979) Penetration of oximes across the blood-brain-barrier. A histochemical study of the cerebral cholinesterases reactivation. Experientia 35:531–532
Maresch W (1957) Die Vergiftung durch Phosphorsdureester. Arch Toxicol 16:285–319
Meiniel R (1971) Etudes histochemiques du comportement du tissu adrénal et de quelques organes riches en glycogène après traitment de l'embryon de Poulet au parathion et à la réserpine. C R Soc Biol (Paris) 165:1918–1921
Meiniel R (1972) Activité acétylcholinestéraseique surrénalienne chez l'embryon de Poulet ágé, dans les conditions normales et après intoxication par le parathion (E 605). C R Soc Biol (Paris) 166:1293–1296
Moeschlin S (1980) Klinik und Therapie der Vergiftungen. Thieme, Stuttgart
Nakata T, Nishijima S, Akai M (1971) Combined staining by thiolacetic acid and Bielschowsky methods. Stain Technol 46:151–153
Neal RA (1967a) Studies on the metabolism of diethyl-4-nitrophenyl phosphorothionate (parathion) in vitro. Biochem J 103:183–191
Neal RA (1967b) Studies of the enzymic mechanism of the metabolism of diethyl-4-nitrophenyl phosphorothionate (parathion) by rat liver microsomes. Biochem J 105:289–297
O'Brien RD (1960) Toxic phosphorous esters. Academic Press, New York London
Oehmichen M (1980) Enzyme alterations in brain tissue during the early postmortal interval with reference to the histomorphology: Review of the literature. Z Rechtsmed 85:81–95
Olajos EJ, Caprio AP de, Rosenblum I (1978) Central and peripheral neurotoxic esterase activity and dose-response relationship in adults hens after acute and chronic oral administration of diisopropyl fluorophosphate. Ecotoxicol Environ Safety 2:383–399
Pearse AGE (1968) Histochemistry, theoretical and applied. Churchill, London
Petty CS, Moore EJ (1958) Histochemical demonstration of cholinesterase. Application to forensic pathology. J Forensic Sci 3:510–520
Petty CS, Lovell MP, Moore EJ (1958) Organic phosphorus insecticides and post-mortem cholinesterase levels. J Forensic Sci 3:226–237
Pribilla O (1954) Vergiftungen mit E605 (0,0-Diäthyl-0, p-nitrophenylthiophosphosäureester). Arch Toxicol 15:210–284
Schrader G (1963) Die Entwicklung neuer insektizider Phosphorsäure-Ester. Verlag Chemie, Weinheim
Wecker L, Dettbarn WD (1976) Paraoxon-induced myopathy: muscle specificity and acetylcholin involvement. Exp Neurol 51:281–291
Wecker L, Mobley PL, Dettbarn WD (1977) Central cholinergic mechanisms underlying adaptation to reduced cholinesterase activity. Biochem Pharmacol 26:633–637
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. J. Peiffer on occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Oehmichen, M., Besserer, K. Forensic significance of acetylcholine esterase histochemistry in organophosphate intoxication. Z Rechtsmed 89, 149–165 (1982). https://doi.org/10.1007/BF01873797
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01873797