Summary
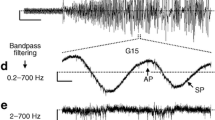
Two microelectrodes have been used to measure membrane potentials simultaneously in pairs of mouse pancreatic islet cells. In the presence of glucose at concentrations between 5.6 and 22.2mm, injection of currenti into cell 1 caused a membrane potential change in this cell,V 1, and, provided the second microelectrode was less than 35 μm away, in a second impaled cell 2,V 2. This result establishes that there is electrical coupling between islet cells and suggests that the space constant of the coupling ratio within the islet tissue is of the order of a few β-cell diameters. The current-membrane potential curvesi-V 1 andi-V 2 are very similar. By exchange of the roles of the microelectrodes, no evidence of rectification of the current through the intercellular pathways was found. Removal of glucose caused a rapid decrease in the coupling ratioV 2 /V 1 . In steady-state conditions, the coupling ratio increases with the concentration of glucose in the range from 0 up to 22mm. Values of the equivalent resistance of the junctional and nonjunctional membranes have been estimated and found to change with the concentration of glucose. Externally applied mitochondrial blockers induced a moderate increase in the junctional resistance possibly mediated by an increase in intracellular Ca2+.
Similar content being viewed by others
References
Ashcroft, S.J.H., Weerasinghe, L.C.C., Randle, P.J. 1973. Interelationship of islet metabolism, adenosine triphosphate content and insulin release.Biochem. J. 132:223–231
Atwater, I., Dawson, C.M., Eddlestone, G.T., Rojas, E. 1981. Voltage noise measurements across the pancreatic β-cell membrane: Calcium channel characteristics.J. Physiol. (London) 314:195–212
Atwater, I., Dawson, C.M., Ribalet, B., Rojas, E. 1979a. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic β-cell.J. Physiol. (London) 288:575–588
Atwater, I., Dawson, C.M., Scott, A., Eddlestone, G.T., Rojas, E. 1980. The nature of the oscillatory behaviour in electrical activity from pancreatic β-cell,Horm. Metab. Res. Suppl. 10:100–107
Atwater, I., Ribalet, B., Rojas, E. 1978. Cyclic changes in potential and resistance of the β-cell membrane induced by glucose in islets of Langerhans from mouse.J. Physiol. (London) 278:117–139
Atwater, I., Ribalet, B., Rojas, E. 1979b. Mouse pancreatic β-cells: Tetraethylammonium blockage of the potassium permeability increase induced by depolarization.J. Physiol. (London) 288:561–574
Dean, P.M. 1973. Ultrastructural morphomotry of the pancreatic β-cell.Diabetologia 9:115–119
Dean, P.M., Matthews, E.K. 1968. Electrical activity in pancreatic islet cells.Nature (London) 219:389–390
Dean, P.M., Matthews, E.K. 1970a. Glucose induced electrical activity in pancreatic islet cells.J. Physiol. (London) 210:255–264
Dean, P.M., Matthews, E.K. 1970b. Electrical activity in pancreatic islet cells: Effects of ions.J. Physiol. (London) 210:265–275
Eddlestone, G.T., Rojas, E. 1980. Evidence of electrical coupling between mouse pancreatic B-cells.J. Physiol. (London) 303:76–77P
Hellman, B., Honkanen, T., Gylfe, E. 1982. Glucose inhibits insulin release induced by Na+ mobilization of intracellular calcium.FEBS Lett. 148:289–292
Jack, J.J.B., Noble, D., Tsien, R.W. 1975. Electric current flow in excitable cells. Clarendon Press, Oxford
Kohen, E., Kohen, C., Thorell, B., Mintz, D.H., Rabinovitch, A. 1979. Intercellular communication in pancreatic islet monolayer cultures: A microfluorometric study.Science 204:862–865
Loewenstein, W.R. 1981. Junctional intercellular communication: The cell-to-cell membrane.Physiol. Rev. 61:829–913
Loewenstein, W.R., Kanno, Y. 1964. Studies on epithelial (gland) cell junction. I. Modification of surface membrane permeability.J. Cell Biol. 22:565–586
Matthews, E.K., Sakamoto, Y. 1975a. Electrical characteristics of pancreatic islet cells.J. Physiol. (London) 246:421–437
Matthews, E.K., Sakamoto, Y. 1975b. Pancreatic islet cells: Electrogenic and electrodiffusional control of membrane potential.J. Physiol. (London) 246:439–457
Meda, P., Amherdt, M., Perrelet, A., Orci, L. 1979. Coupling between insulin-containing cells. A morphological approach.Int. Cong. Series No. 500, Diabetes. W.K. Waldausl, editor. Excerpta Medica, North-Holland
Meda, P., Denef, J.F., Perrelet, A., Orci, L. 1980a. Nonrandom distribution of gap junctions between pancreatic β-cells.Am. J. Physiol. 238:C114-C119
Meda, P., Halban, P., Perrelet, A., Renold, A.E., Orci, L. 1980b. Gap junction development is correlated with insulin content in the pancreatic β-cell.Science 209:1026–1028
Meda, P., Perrelet, A., Orci, L. 1979. Increase of gap junctions between pancreatic β-cells during stimulation of insulin secretion.J. Cell Biol. 82:441–448
Meda, P., Perrelet, A., Orci, L. 1980c. Gap junctions and β-cell function.Horm. Metab. Res. Suppl. 10:157–162
Meissner, H.P. 1976a Electrical characteristics of the pancreatic β-cell.J. Physiol. (Paris) 72:757–767
Meissner, H.P. 1976b. Electrophysiological evidence for coupling between β-cells of pancreatic islets.Nature (London) 262:502–504
Meissner, H.P., Atwater, I. 1975. The kinetics of electrical activity of B-cells in response to a square wave stimulation with glucose and libenclamide.Horm. Metab. Res. 8:11–15
Michaels, R.L., Sheridan, J.D. 1981. Islets of Langerhans: Dye coupling among immunocytochemically distinct cell types.Science 214:801–803
Orci, L. 1974. A portrait of the pancreatic β-cell.Diabetologia 10:163–187
Orci, L., Unger, R.H., Renold, A.E. 1973. Structural coupling between pancreatic islet cells.Experientia 29:1015–1018
Ribalet, B., Beigelman, P.M. 1979. Cyclic variation of K+ conductance in pancreatic β-cells: Ca2+ and voltage dependence.Am. J. Physiol. 237:C137-C146
Ribalet, B., Beigelman, P.M. 1980. Calcium action potentials and potassium permeability activation in pancreatic β-cells.Am. J. Physiol. 239:C124-C133
Rojas, E., Hidalgo, C. 1968. Effect of temperature and metabolic inhibitors on45Ca outflow from squid giant axons.Biochim. Biophys. Acta 163:550–556
Rose, B., Loewenstein, W.R. 1975. Calcium ion distribution in cytoplasm visualized by aequorin. Distribution in the cytosol is restricted due to energized sequestering.Science 190:1204–1206
Rose, B., Loewenstein, W.R. 1976. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: A study with aequorin.J. Membrane Biol. 28:87–119
Rose, B., Rick, R. 1978. Intracellular pH, intracellular free Ca, and permeability of cell junction.J. Membrane Biol. 44:377–415
Rose, B., Simpson, I., Loewenstein, W.R. 1977. Calcium ion produces graded changes in permeability of membrane channels in cell junction.Nature (London) 267:625–627
Scott, A.M., Atwater, I., Rojas, E. 1981. A method for the simultaneous measurement of insulin release on β-cell membrane potential in single mouse islets of Langerhans.Diabetalogia 21:470–475
Simpson, I., Rose, B., Loewenstein, W.R. 1977. Size limit of molecules permeating the junctional membrane channels.Science 195:294–296
Sugden, M.C., Ashcroft, S.J.H. 1978. Effects of phosphoenolpyruvate, other glycolytic intermediates and methylxanthines on calcium uptake by a mitochondrial fraction from rat pancreatic islets.Diabetologia 15:173–180
Turin, L., Warner, A.E. 1977. Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo.Nature (London) 270:56–57
Turin, L., Warner, A. 1980. Intercellular pH in earlyXenopus embryos: Its effect on current flow between blastomeres.J. Physiol. (London) 300:489–504
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eddlestone, G.T., Gonçalves, A., Bangham, J.A. et al. Electrical coupling between cells in islets of langerhans from mouse. J. Membrain Biol. 77, 1–14 (1984). https://doi.org/10.1007/BF01871095
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01871095