Summary
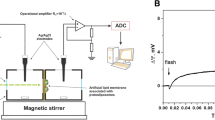
A suspension of purple membrane fragments in a solution of soya phosphatidyl-choline in hexane is spread at an air-water interface. Surface pressure and surface potential measurements indicate that the membrane fragments and lipids organize at the interface as an insoluble film. Electron microscopy of shadow-cast replicas of the film reveal that in the bacteriorhodopsin to soya PC weight ratio range of 2∶1 to 10∶1, these films consist of nonoverlapping membrane fragments which occupy approximately 35% of the surface area and are separated by a lipid monolayer. Furthermore, the membrane fragments are oriented, with their intracellular surface towards the aqueous subphase. Nearly all the bacteriorhodopsin molecules at the interface are spectroscopically intact and exhibit visible spectral characteristics identical to those in aqueous suspensions of purple membrane and in intact bacteria. In addition, bacteriorhodopsin in air-dried interface films show spectral changes upon dark-adaptation and upon flash illumination similar to those observed in aqueous suspensions of purple membrane, but with slower kinetics. The kinetics of the spectral changes in interface films can be made nearly the same as in aqueous suspension by immersing the films in water.
Similar content being viewed by others
References
Adams, D.J., Evans, M.T.A., Mitchell, J.R., Phillips, M.C., Rees, P.M. 1971. Adsorption of lysozyme and some acetyl derivatives at the air-water interface.J. Polym. Sci. Part C 34:167
Blaurock, A.E. 1975. Bacteriorhodopsin: A transmembrane pump containing α-helix.J. Mol. Biol. 93:139
Blaurock, A.E., Stoeckenius, W. 1971. Structure of the purple membrane.Nature New Biol. 233:152
Bligh, E.G., Dyer, W.J. 1959. A rapid method of total lipid extraction and purification.Can. J. Biochem. 37:911
Blodgett, K.B. 1935. Films built by depositing successive monomolecular layers on a solid surface.J. Am. Chem. Soc. 57:1007
Bogomolni, R.A., Hwang, S.-B. Tseng, Y.-W., King, G.I., Stoeckenius, W. 1977. Orientation of the bacteriorhodopsin transition dipole.,Biophys. Soc. (Abstr.) 98a
Boguslavsky, L.I., Kondrshin, A.A., Kozlov, I.A., Metelsky, S.T., Skulachev, V.P., Volkov, A.G. 1975. Charge transfer between water and octane phases by soluble mitochondrial ATPase (F1), bacteriorhodopsin and respiratory chain enzymes.FEBS Lett. 50:223
Bridgen, J., Walker, I.D. 1976. Photoreceptor protein from the purple membrane ofHalobacterium halobium: Molecular weight and retinal binding site.Biochemistry 15:792
Danon, A., Stoeckenius, W. 1974. Photophosphorylation inHalobacterium halobium.Proc. Nat. Acad. Sci. USA 71:1234
Das, M.L., Crane, F.L. 1964. Proteolipids. I. Formation of phospholipid-cytochrome c complexes.Biochemistry 3:696
Dencher, N., Wilms, M. 1975. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin.Biophys. Struct. Mechanism 1:259
Drachev, L.A., Frolov, V.N., Kaulen, A.D., Liberman, E.A., Ostrumov, S.A., Plankunova, V.G., Semenov, A.Y., Skulachev, V.P. 1976. Reconstitution of biological molecular generators of electric current.J. Biol. Chem. 251:7059
Fisher, K.A. 1975. “Half” membrane enrichment: Verification be electron microscopy.Science 190:983
Gaines G.L., Jr. 1966. Insoluble Monolayers at Liquid-Gas Interfaces. Interscience, New York
Gitler, C., Montal, M. 1972. Thin-proteolipid films: A new approach to the reconstitution of biological membranes.Biochem. Biophys. Res. Commun. 47:1486
Goerke, J., Harper, H.H., Borowitz, M. 1970. The interaction of calcium with monolayers of stearic acid.In: Surface Chemistry of Biological System. M. Blank, editor. Plenum Press, New York-London
Hanahan, D.J., Dittmer, J.C., Warashina, E. 1957. A column chromatographic separation of classes of phospholipids.J. Biol. Chem. 228:685
Henderson, R. 1975. The structure of the purple membrane fromHalobacterium halobium: Analysis of the X-ray diffraction pattern.J. Mol. Biol. 93:123
Henderson, R., Unwin, P.N.T. 1975. Three dimensional model of purple membrane obtained by electron microscopy.Nature (London) 257:28
Hwang, S.-B., Korenbrot, J.I., Stoeckenius, W. 1977. Proton transport by bacteriorhodopsin through an interface film.J. Membrane Biol. 36:137
Hwang, S.-B., Stoeckenius, W. 1977. Purple membrane vesicles: Morphology and proton translocation.J. Membrane Biol. 33:325
James, L.K., Augenstein, L.G. 1966. Adsorption of enzymes at interfaces: Film formation and the effect on activity.Adv. Enzymol. 28:1
Kriebel, A.N., Albrecht, A.C. 1976. Excitonic interaction among three chromophores: An application to the purple membrane ofHalobacterium halobium.J. Chem. Phys. 65:4575
Kung, M. Chu, Devault, D., Hess, B., Oesterhelt, D. 1975. Photolysis of bacterial rhodopsin.Biophys. J. 15:907
Kushwaha, S.C., Kates, M., Martin, W.G. 1975. Characterization and composition of the purple membrane and red membrane fromHalobacterium cutirubrum.Can. J. Biochem. 53:284
Liebman, P.A. 1962. In situ microspectrophotometric studies on the pigments of single retinal rods.Biophys. J. 2:161
Loeb, G.I. 1971. Spectroscopy of protein monolayers: A transition in β-lactoglobulin films.J. Polym. Sci. Part C 34:63
Lozier, R.H., Bogomolni, R.A., Stoeckenius, W. 1975. Bacteriorhodopsin: A light proton pump inH. halobium.Biophys. J. 15:955
Lozier, R.H., Niederberger, W., Bogomolni, R.A., Hwang, S.-B., Stoeckenius, W. 1976. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments,H. halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane.Biochim. Biophys. Acta 440:545
Malcolm, B.R. 1968. Molecular structure and deuterium exchange in monolayers of synthetic polypeptides.Proc. Roy. Soc. A 305:363
Mao, B., Becher, B., Kilbride, P., Ebrey, T.G., Honig, B. 1977. Exciton, interaction and chromophore orientation in the purple membrane.Biophys. Soc. (Abstr.) 76a
Miller, I.R., Bach, D. 1973. Biopolymers at Interfaces.In: Surface and Colloid Sci. Vol. 6, p. 185. E. Matijevic, editor. John Wiley and Sons, New York
Mitchell, P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism.Nature (London) 191:144
Montal, M., Mueller, P. 1972. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties.Proc. Nat. Acad. Sci. U.S.A. 69:3561
Oesterhalt, D. 1975. The purple membrane ofHalobacterium halobium: A new system for light energy conversion. Ciba Foundation Symposium 31 (new series). p.147. Elsevier, Amsterdam
Oesterhalt, D., Hess, B. 1973. Reversible photolysis of the purple complex in the purple membrane ofH. halobium.Eur. J. Biochem. 37:316
Oesterhelt, D., Stoeckenius, W. 1973. Function of a new photoreceptor membrane.Proc. Nat. Acad. Sci. USA 70:2853
Oesterhelt, D., Stoeckenius, W. 1974. Isolation of the cell membrane ofH. halobium and its fraction into red and purple membrane.In: Methods in Enzymology, Biomembranes Vol. 31. S. Fleischer and R. Estabrook, editors. Academic Press, New York
Racker, E., Stoeckenius, W. 1974. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation.J. Biol. Chem. 249:662
Renthal, R., Lanyi, J.K. 1976. Light-induced membrane potential and pH gradient inHalobacterium halobium envelope vesicles.Biochemistry 15:2136
Tsofina, L.M., Liberman, E.A., Babakov, A.V. 1966. Production of bimolecular protein-lipid membranes in aqueous solution.Nature (London) 212:681
Wald, G., Durell, J., St. George, R.C.C. 1950. The light reaction in the bleaching of rhodopsin.Science 17:179
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hwang, SB., Korenbrot, J.I. & Stoeckenius, W. Structural and spectroscopic characteristics of bacteriorhodopsin in air-water interface films. J. Membrain Biol. 36, 115–135 (1977). https://doi.org/10.1007/BF01868147
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01868147