Summary
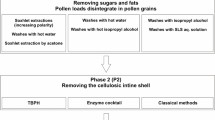
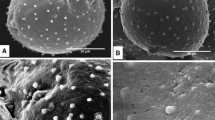
Methods for the removal of exine from mature, ungerminatedLilium longiflorum pollen and release of intact gametophytes (sporoplasts) have been developed. These methods rely on the low temperature solvolytic activity of 4-methylmorpholine N-oxide (MMNO), which allows partial or complete detachment of exine from intine during subsequent washing procedures. These methods are: aqueous MMNO combined with cyclohexylamine (method I), aqueous MMNO at alkaline pH (method II), and aqueous MMNO containing a high Ca2+ concentration with added cellulysin and macerase (method III). Sporoplasts produced by methods I and II are most frequently completely separated from exine and, as shown by histochemical tests, enveloped by the intine layer. Selected enzyme activities in method II sporoplasts are measurable but, as indicated by other tests, considerable damage to the plasma membrane accompanies this treatment. Sporoplasts produced by melhod III largely remain attached to their ruptured exine layer and retain substantial biological competence in terms of extractable enzyme activities, membrane integrity, and respiration.
Similar content being viewed by others
Abbreviations
- MMNO:
-
4-methylmorpholine N-oxide
- SEM:
-
scanning electron microscope
- TEM:
-
transmission electron microscope
References
Bajaj YPS (1974) Isolation and culture studies on pollen tetrad and pollen mother-cell protoplasts. Plant Sci Lett 3: 93–99
— (1983)In vitro production of haploids. In:Evans D, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture, vol 1, Techniques for propagation and breeding. Macmillan Pub Co, New York, pp 228–287
—,Davey MR (1974) The isolation and ultrastructure of pollen protoplasts. In:Linskens HF (ed) Fertilization in higher plants. North Holland Pub Co, Amsterdam, pp 73–80
Baldi BG, Franceschi VR, Loewus FA (1986 a) Dissolution of pollen intine and release of sporoplasts. In:Mulcahy DL, BerGamini Mulcahy G, Ottaviano E (eds) Biotechnology and ecology of pollen. Springer, New York Berlin Heidelberg Tokyo, pp 77–82
—,Weeks W, Loewus FA (1986 b) Release of intine-clad sporoplasts from pollen exine at low temperature. Plant Physiol 80 [suppl] 76 abst 400
—,Franceschi VR, Loewus FA (1987) Localization of phosphorus and cation reserves inLilium longiflorum pollen. Plant Physiol 83: 1018–1021
Bhojwani SS, Cocking EC (1972) Isolation of protoplasts from pollen tetrads. Nature New Biol 239: 29–30
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Chanzy H, Chumpitazi B, Peguy A (1980) Solutions of polysaccharides in N-methylmorpholine N-oxide (MMNO). Carbohydr Polym 2: 35–42
—,Dube M, Marchessault RH (1982) Crystallization of cellulose with N-methylmorpholine N-oxide: a new method of texturing cellulose. J Polym Sci: Polym Lett 17: 219–226
Coleman A, Goff LJ (1985) Application of fluorochromes to pollen biology. I. Mithramycin and 4′-6-diamino-2-phenylindole (DAPI) as vital stains and for quantitation of nuclear DNA. Stain Tech 60: 145–151
Davis B (1985) Factors influencing protoplast isolation. In:Peberdy JF, Ferenczy L (eds) Fungal protoplasts. Marcel Dekker Inc, New York, pp 45–71
Dickinson DB (1968) Rapid starch synthesis associated with increased respiration in germinating lily pollen. Plant Physiol 43: 1–8
— (1978) Influence of borate and pentaerythritol concentrations on germination and tube growth ofLilium longiflorum pollen. J Am Soc Hort Sci 103: 413–416
—,Lin JJ (1986) Phytases of germinating lily pollen. In:Mulcahy DL, Bergamini Mulcahy G, Ottaviano E (eds) Biotechnology and ecology of pollen. Springer, New York Berlin Heidelberg Tokyo, pp 357–362
Duhoux E (1980) Protoplast isolation of gymnosperm pollen. Z Pflanzenphysiol 99: 207–214
Evans DA, Bravo JE (1983) Protoplast isolation and culture. In:Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant tissue culture, vol1. Macmillan Publ Co, New York, pp 124–176
Franceschi VR, Horner Jr HT (1979) Nuclear condition of the anther tapetum ofOrnithogalum caudatum during microsporogenesis. Cytobiologie 18: 413–421
Gagnaire D, Mancier D, Vincendon M (1980) Cellulose organic solutions: A nuclear magnetic resonance investigation. J Polym Sci: Polym Lett 18: 13–25
Galun E (1981) Plant protoplasts as physiological tools. Ann Rev Plant Physiol 32: 237–266
Garen A, Levinthal C (1980) A fine-structure genetic and chemical study of the enzyme alkaline phosphatase ofEscherichia coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta 38: 470–483
Guillard RRL (1973) Division rates. In:Stein JR (ed) Handbook of phycological methods. Cambridge Univ Press, Cambridge, England, pp 289–311
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Tech 45: 115–120
Huang C-N, Cornejo MJ, Bush DS, Jones RL (1986) Estimating viability of plant protoplasts using double and single staining. Protoplasma 135: 80–87
Jensen WA (1962) Botanical histochemistry. WH Freeman, San Francisco, CA
Lillie RD (1977) HJ Conn's biological stains. Ninth ed, Williams & Wilkins, Baltimore, MD
Loewus FA, Baldi BG, Franceschi VR, Meinert LD, McCoLLum JJ (1985) Pollen sporoplasts: dissolution of pollen walls. Plant Physiol 78: 652–654
Loewus MW, Loewus FA (1982) myo-Inositol-1-phosphatase from the pollen ofLilium longiflorum Thunb. Plant Physiol 70: 765–770
Power JB (1973) Isolation of mature tobacco pollen protoplasts. In:Street HE (ed) Plant tissue and cell culture. Univ Calif Press, Berkeley, pp 118–120
Rajkumar TV, Woodtin BM, Rutter WJ (1966) Fructose diphosphate aldolase. III. Aldolase B from (adult) rabbit liver. Methods Enzymol 9: 491–498
Rosen BH, Berliner MD, Petro MJ (1985) Protoplast induction inChlorella pyrenoidosa. Plant Sci 41: 23–30
Scott JJ, Loewus FA (1986) A calcium-activated phytase from pollen ofLilium longiflorum. Plant Physiol 82: 333–335
Singh MB, Knox RB (1984) Invertases ofLilium pollen: characterization and activity duringin vitro germination. Plant Physiol 74: 510–515
Southworth D (1974) Solubility of pollen exines. Am J Bot 61: 36–44
— (1985) Pollen exine substructure. I.Lilium longiflorum. Am J Bot 72: 1274–1283
Takegami MH, Ito M (1975) Studies on the behavior of meiotic protoplasts. III. Features of nuclear and cell division for lily protoplasts in the cell-wall-free state. Bot Mag Tokyo 88: 187–196
Thompson SW (1966) Selected histochemical and histopathological methods. C C Thomas, Springfield, Ill
Yang HY (1986) Fluorescein diacetate used as a vital stain for labeling living pollen tubes. Plant Sci 44: 59–63
Zhu C, Xie Y, Hu S (1983) Isolation anf cultural behavior of pollen tube subprotoplasts inAntirrhinum majus L. Acta Bot Sinica 26: 459–465
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baldi, B.G., Franceschi, V.R. & Loewus, F.A. Preparation and properties of pollen sporoplasts. Protoplasma 141, 47–55 (1987). https://doi.org/10.1007/BF01276787
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01276787