Abstract
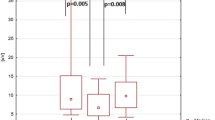
It has previously been shown that 0.6 mg of scopolamine produces a delay in the flash visual evoked potential of young normal volunteers, while the pattern-reversal response does not change in latency. Recent work has shown that this drug differentially affects parvocellular and magnocellular systems. To investigate this effect, two studies were performed. In the first study, 0.4 mg of scopolamine was injected intramuscularly into 11 young, healthy male volunteers who had fasted overnight. The visual evoked potential was recorded to both binocular flash stimulation and monocular pattern-reversal stimulation by means of a checkerboard consisting of 56′ checks in a 28° field. Responses were recorded before administration of the drug and then 1, 2, 4 and 6 hours after administration. The scopolamine produced a slowing of the flash P2 latency of approximately 6 ms (p < 0.05) two hours after drug administration. There was no effect on the latency of the flash N2 or pattern-reversal N75 or P100. There was an increase in amplitude of the flash N2-P2 component 6 hours after drug administration and an increase in the amplitude of the N75 and P100 2, 4 and 6 hours after the drug. Further subjects were investigated with the use of topical administration of 0.125% scopolamine applied monocularly. In all studies the other eye acted as a control. The subjects were again young healthy volunteers. The visual evoked potential was recorded to both flash and pattern-reversal stimulation with a checkerboard consisting of 60′ checks counterphasing at 2 Hz within a 5° field. Results suggest that systemic scopolamine affects the tectal pathway but has no peripheral effect.
Similar content being viewed by others
References
Harding GFA, Doggett A, Orwin A, Smith EJ. Visual evoked potentials in pre-senile dementia. Doc Ophthalmol Proc Series 1981; 27: 193–201.
Cosi V, Vitelli E, Gozzoli A, Corona M, Ceroni M, Callieco R. Visual evoked potentials in aging of the brain. In: Courjon J, Mauguiere F, Revol M. eds. Clinical applications of evoked potentials in neurology. New York: Raven Press, 1982.
Visser SL, Van Tilburg W, Hooijer C, Jonker C, De Rijke W. Visual evoked potentials (VEPs) in senile dementia (Alzheimer type) and in non-organic behavioural in the elderly. Comparison with EEG parameters. Electroencephalogr Clin Neurophysiol 1985; 60: 115–21.
Coben LA, Danzuger WL, Hughes CP. Visual evoked potentials in mild senile dementia of Alzheimer type. Electroence Clin Neurophysiol 1983; 55: 121–30.
Wright CE, Harding GFA, Orwin A. Presenile dementia. The use of the flash and pattern VEP in diagnosis. Electroence Clin Nevrophysiol 1984; 57: 405–15.
Danesi MA, Huxley P, Murray NMF. Flash and pattern VEPs in dementia. Electroencephalogr Clin Neurophysiol 1985; 61: S196.
Wright CE, Harding GFA, Orwin A. The flash and pattern VEP as a diagnostic indicator of dementia. Doc Ophthalmol 1986; 62: 89–96.
Katz B, Rimmer S, Iragui V, Katzman R. Abnormal pattern electroretinogram in Alzheimer's disease. Evidence for retinal ganglion cell degeneration. Ann Neurol 1989; 26: 221–5.
Philpot MP, Amin D, Levy R. Visual evoked potentials in Alzheimer's disease. Correlations with age and severity. Electroencephalogr Clin Neurophysiol 1990; 77: 323–9.
Harding GFA, Wright CE. Visual evoked potentials in acute optic neuritis. In: Hess RF, Plant GT, eds. Cambridge, England: Cambridge Universrty Press, Optic neuritis. 1986; 230–54.
Spehlmann R. Acetylcholine and the synaptic transmission of non-specific impulses to the visual cortex. Brain 1971; 94: 139–50.
Spehlmann R, Daniels JC, Smathers C. Acetylcholine and the synaptic transmission of specific impulses to the visual cortex. Brain 1971; 94: 125–38.
Shute CCD, Lewis PR. The ascending cholinergic reticular system. Neurocortical olfactory and subcortical projections. Brain 1967; 90: 497–519.
Harding GFA, Dhanesha U. The visual evoked subcortical potential to pattern reversal stimulation. Electroencphalogr Clin Neurophysiol 1985; 6: S136.
Humphrey NK. Responses to visual stimuli of units in the superior colliculi of rats and monkeys. Exp Neurol 1968; 20: 312–40.
Cyander M, Berman N. Receptive field organization of monkey superior colliculus. J Neurophysiol 1972; 35: 187–201.
Schiller PH, True SD, Conway JL. Paired stimulation of the frontal eye fields and superior colliculus of rhesus monkey. Brain Res 1979; 179: 162–4.
Wirtz RH, Albano JE. Visual motor function of the primate superior colliculus. Annu Rev Neurosci 1980; 3: 189–226.
Spellman R, Gross RA, Ho SU, Leestra JE, Norcross KA. Visual evoked potential and postmortem findings in a case of cortical blindness. Ann Neurol 1977; 2: 531–4.
Celesia GG, Bushnell D, Toleihis SC, Brigell MG. Cortical blindness and reduced vision. Is the “second” visual system in humans capable of more than rudimentary visual perception? Neurology 1991; 41: 862–9.
Schlotterer G, Moscovitch M, McLachlan C. Visual processing deficits as assessed by spatial frequency contrast sensitivity and backward masking in normal aging and Alzheimers disease. Brain 1983; 107: 309–25.
Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques In Alzheimer's disease. A quantitative study of visual and auditory cortices. J Neurosci 1987; 7: 1799–1808.
Mann DMA, Tucker CM, Yates PO. The topographic distribution of senile plaques and neurofibrillary tangles in the brains of non demented persons of different ages. Neuropathol Appl Neurobiol 1987; 13: 123–39.
Bajalan AAA, Wright CE, Van der Vliet VJ. Changes in the human visual evoked potential caused by the anticholinergic agent hyoscine hydrobromide. Comparison with results in Alzheimer's disease. J Neurol Neurosurg Psych 1986; 49: 175–81.
Ostfeld AM, Arguete A. Central nervous system effects of hyoscine in man. Pharmacol Exp Ther 1962; 137: 133–9.
Crow TJ, Grove-White IG. An analysis of the learning deficit following hyoscine administration in man. Br J Pharmacol 1973; 49: 322–7.
Drachman DA, Leavitt J. Human memory and the cholinergic system. Arch Neurol 1974; 30: 113–21.
Drachman DA. Memory, dementia, and the cholinergic system. In: Katzman R, Terry RD, Bick KL, eds. Alzheimer's disease, senile dementias and related disorders. New York: Raven Press, 1978: 141–8.
Sitaram N, Weingartner H, Gillin JC. Human serial learning. Enhancement with are-choline and impairment with scopolamine. Science 1978; 201: 274–6.
Davis KL, Mohs RC, Tinklenberg JR, Pfefferbaum A, Hollister LE, Kopell BS. Physostigmine. Improvement of long-term memory processes in normal humans. Science 1978; 201: 272–4.
Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. J Neurol Sci 1977; 34: 247–65.
Davis P. Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res 1979; 171: 319–27.
Rossor MN, Garrett NJ, Johnson AL, Mountjoy CQ, Roth M, Iversen LL. A post-mortem study of the cholinergic and GABA systems in senile dementia. Brain 1982; 105: 313–30.
Gilles G, De Buyl O, Genevrois C, Salma M, Mendlewicz J. Specificity of visual evoked potentials alterations in Alzheimer's disease. Comparison with normal aging depression and scopolamine administration in young healthy volunteers. Acta Neurol Belg 1989; 89: 226–7.
Sannita WG, Fioretto M, Maggi L, Rosadini G. Effects of scopolamine parenteral administration on the eletroretinogram, visual evoked potentials, and quantitative electroencephalogram of healthy volunteers. Doc Ophthalmol 1988; 67: 379–88.
Sadun AA, Bassi CJ. The visual system in Alzheimer's disease. In: Cohen B, Bodis-Wollner I, eds. Vision and the brain. New York: Raven Press, 1990: 331–47.
Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic nerve degeneration in Alzheimer's disease. N Engl J Med 1986; 315: 485–7.
Morrison JD, Reilly J. The effects of 0.025% hyoscine hydrobromide on visual function in man. Ophthalmol Physiol Opt 1989; 9: 41–5.
Kaplan E, Lew B, Shapley RM. New views of primate retinal function. In: Osborne N, ed. Progress in retinal research. 1991; 9: 273–336, Oxford, Pergamon Press.
Seddon JM, Sahagian CR, Glynn RJ, Sperduto RD, Gragoudas ES, Eye Disorders Case-Control Study Group. Evaluation of an iris color classification system. Invest Ophalmol Vis Sci 1990; 31: 1592–1598.
O'Connor Davies PH, ed. The action and uses of ophthalmic drugs. 2nd ed. London: Butterworths.
Ray PG Meador KJ Lorin DW, Murro AM, Buccafuso JJ, Yan X-H, Zamrini EY, Thompson WO, Thompson EE. Effects of scopolamine on visual evoked potentials in aging and dementia. Electroencephalogr Clin Neurophysiol 1991; 80: 347–51.
Smith AT, Early F, Jones GH. Comparison of the effects of Alzheimer's disease, normal aging and scopolamine on human transient visual evoked potentials. Psychopharmacology 1990; 102: 535–43.
Wright CE, Williams DE, Drasdo N, Harding GFA. The influence of age on the electroretinogram and visual evoked potential. Doc Ophthalmol 1985; 59: 365–84.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harding, G.F.A., Daniels, R., Panchal, S. et al. Visual evoked potentials to flash and pattern reversal stimulation after administration of systemic or topical scopolamine. Doc Ophthalmol 86, 311–323 (1994). https://doi.org/10.1007/BF01203554
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01203554