Abstract
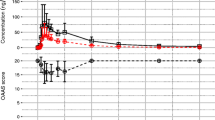
Healthy human subjects received single and multiple oral doses of flunitrazepam. Absorption and disposition were first order and reproducible from administration to administration. The oral doses were virtually completely available to the liver, and elimination from the body occurred entirely via metabolism. Assuming the liver to be the sole eliminating organ, hepatic blood clearance and extraction ratio were approximately 0.235 liter/hr/kg and 0.154, respectively. Steady-state blood volume of distribution averaged 3.76 liters/kg in the single-dose studies. Terminal exponential half-lives from the single- and multiple-dose studies (different subjects) averaged 13.5 and 19.2 hr, respectively, these differences were not due to clearance changes but were entirely attributable to variations in volumes of distribution.
Similar content being viewed by others
References
G. Wendt. Schicksal des Hypnotikums Flunitrazepam im menschlichen Organismus. In W. Hügin, G. Hossli, and M. Gemperle (eds.),Bisherige Erfahrungen mit “Rohypnol” (Flunitrazepam) in der Anästhesiologie und Intensivtherapie, F. Hoffman-La Roche & Co. A.G., Basel, 1976, pp. 27–38.
R. Amrein, J.-P. Cano, and W. Hügin. Pharmakokinetische und pharmakodynamische Befunde nach einmaliger intravenöser, intramuskulärer und oraler applikation von “Rolypnol.” In W. Hügin, G. Hossli, and M. Gemperle (eds),Bisherige Erfahrungen mit “Rohypnol” (Flunitrazepam) in der Anästhesiologie und Intensivtherapie, F. Hoffmann-La Roche & Co. A.G., Basel, 1976, pp. 39–56.
H. G. Boxenbaum. Data on file, Hoffmann-La Roche Inc., Nutley, N.J.
S. A. Kaplan and S. Cotler. Use of cannulated everted intestinal sac for serial sampling as a drug absorbability (permeability) screen.J. Pharm. Sci. 61:1361–1365 (1972).
J. H. Wood, J. E. Syarto, and H. Letterman. Improved holder for intrinsic dissolution rate studies.J. Pharm. Sci. 54: 1068 (1965).
J. A. de Silva and I. Bekersky. Determination of clonazepam and flunitrazepam in blood by electron-capture gas-liquid chromatography.J. Chromatog. 99:447–460 (1974).
J. A. F. de Silva, C. V. Puglisi, and N. Munno. Determination of clonazepam and flunitrazepam in blood and urine by electron-capture GLC.J. Pharm. Sci. 63:520–527 (1974).
H. J. Knop, E. van der Kleijn, and L. C. Edmunds. The determination of clonazepam in plasma by gas-liquid chromatography.Pharm. Weekblad 110:297–309 (1975).
J. G. Wagner.Biopharmaceutics and Relevant Pharmacokinetics, Drug Intelligence Publications, Hamilton, Ill., 1971, p. 295.
C. M. Metzler, G. L. Elfring, and A. J. McEwen.A Users Manual for NONLIN and Associated Programs, Upjohn Co., Kalamazoo, Mich., April 20, 1974.
F. B. Eatman, W. A. Colburn, H. G. Boxenbaum, H. N. Posmanter, R. E. Weinfeld, R. Ronfeld, L. Weissman, J. D. Moore, M. Gibaldi, and S. A. Kaplan. Pharmacokinetics of diazepam following multiple dose oral administration to healthy human subjects.J. Pharmacokin. Biopharm. 5:481–494 (1977).
S. Riegelman, J. C. K. Loo, and M. Rowland. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment.J. Pharm. Sci. 57:117–123 (1968).
M. Rowland, T. F. Blaschke, P. J. Meffin, and R. L. Williams. Pharmacokinetics in disease states modifying hepatic and metabolic function. In L. Z. Benet (ed.),The Effect of Disease States on Drug Pharmacokinetics, American Pharmaceutical Association, Washington, D.C., 1976, pp. 53–75.
C. V. Greenway and R. D. Stark. Hepatic vascular bed.Physiol. Rev. 51:23–65 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boxenbaum, H.G., Posmanter, H.N., Macasieb, T. et al. Pharmacokinetics of flunitrazepam following single- and multiple-dose oral administration to healthy human subjects. Journal of Pharmacokinetics and Biopharmaceutics 6, 283–293 (1978). https://doi.org/10.1007/BF01060092
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01060092