Abstract
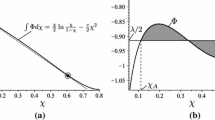
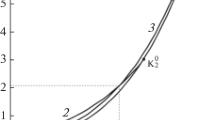
The density-density plot of the critical lines of the van der Waals equation at the van Laar point is analyzed through its algebraic properties. It is shown that this curve is an irreducible expression of the fifth degree of genus one. In addition, we show the topology of the second branch, i.e., theT=0 solution, which will interact with the first branch as soon as the energy parameters are slightly different from the van Laar values. Finally, we analyze the behavior of the van der Waals equation near the point at which liquid-liquid separation takes place.
Similar content being viewed by others
References
R. L. Scott and P. H. van Konynenburg,Disc. Faraday Soc. 49:87 (1970); P. H. van Konynenburg and R. L. Scott,Phil. Trans. R. Soc. 298:495 (1980).
D. Furman and R. B. Griffiths,Phys. Rev. A 17:1139 (1978).
D. J. Korteweg,Arch. Néere. Sci. Ex. Nat. II VII:235 (1903);K. Akad. Amst. 5:445–465 (1903).
A. van Pelt and Th. W. de Loos,J. Chem. Phys. 97:1271–1281 (1992).
A. van Pelt, C. J. Peters, and J. de Swaan Arons,J. Chem. Phys. 95:7569–7575 (1991); A. van Pelt, Critical phenomena in binary fluid mixtures; Classification of phase equilibria with the simplified-perturbed-hard-chain theory, Thesis, Technical University of Delft (1992).
J. J. van Laar,Proc. Sect. Sci. K. Ned. Akad. Wet. 7:646 (1905);8:33 (1905).
E. P. van Emmerik, J. J. van Laar (1860–1938), a mathematical chemist, Thesis, Technical University of Delft (1991).
U. K. Deiters and I. L. Pegg,J. Chem. Phys. 90:6632–6641 (1989).
P. H. E. Meijer, I. L. Pegg, J. Aronson, and M. Keskin, The critical lines of the van der Waals equation for binary mixtures around the van Laar point,Fluid Phase Equilibria 58:65–80 (1990).
P. H. E. Meijer, The van der Waals equation of state around the van Laar point,J. Chem. Phys. 90:448 (1989).
Robert J. Walker,Introduction to Algebraic Curves (Princeton University Press, Princeton, New Jersey); W. Fulton and R. Weiss,Algebraic Curves, An Introduction to Algebraic Geometry (Benjamin/Cummins, Menlo Park, California, 1969).
Th. Kraska and U. K. Deiters,J. Chem. Phys. 96:539 (1992).
J. M. H. Levelt Sengers, Thermodynamics of solutions near the solvent's critical point, inSupercritical Fluid Technology, Th. J. Bruno and J. F. Ely, eds. (CRC Press, Boca Raton, Florida, 1991), Chapter 1.
C. M. Knobler and R. L. Scott, inPhase Transitions and Critical Phenomena, Vol. 9, C. Domb and J. L. Leibowitz, eds. (Academic Press, 1984), p. 163.
F. E. C. Scheffer,Heterogene Evenwichten in Unaire and Binaire Stelseis, 2nd ed. (Uitg. Waltman, Delft, 1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meijer, P.H.E., Levelt, A.H.M. & Miller, B.R. Analysis of the critical line network for the van der Waals equation at the van Laar point. J Stat Phys 71, 299–312 (1993). https://doi.org/10.1007/BF01048101
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01048101