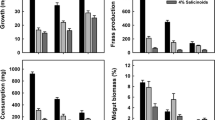
Abstract
A variety of oxidases, reductases, esterases, epoxide hydrolases, and group transferases in herbivorous insects and mites detoxify and facilitate the excretion of toxic phytochemicals (allelochemicals). Current theory indicates that the cytochrome P-450-dependent mixed-function oxidases (MFOs) are by far the most important enzymes because they have many attributes that are essential for an effective detoxification system. Data presented here on the midgut microsomal MFO activity of larvae of the gypsy moth,Lymantria dispar, are discussed in the light of previous work and support the theory. In the gypsy moth, the MFO levels exhibit a parallel trend with changes in specific feeding rates, and changes in the specific activity of the enzyme appear to be regulated ontogenetically and by inductive effect of chemicals in the diet. The specific activity of the MFOs rises more sharply on leaves of a highly preferred type-1 plant, the pin oak, than on an artificial wheat germ diet; the increase from mid-second instar to mid-fifth is 4.5- and 1.8-fold, respectively. The relationship of food consumption rate to increase in body mass (W) was slightly in excess of a 1∶1 ratio for both pin oak and the artificial diet, indicating that the feeding rate surpasses the increase in W (a rare phenomenon in insects). Moreover, the surface-to-volume ratios are fairly constant for combined data of gut lumen and epithelium in second to fifth instars, because the volume occupied by the epithelial cells is much larger than in older ones. Thus, it is concluded that greater specific activity of the MFO is necessary with larval advancement to higher instars in order that they may process dietary allelochemicals with an efficiency comparable to younger larvae. Additional data suggest that MFO level increases reflect further adaptation to: (1) normal, seasonal changes in plants' allelochemical composition and concentration; (2) increase in allelochemical concentration in response to leaf damage; and (3) the risk faced by dispersing larvae of encountering a greater amount and variety of allelochemicals on suboptimal/ less suitable plants. Evidence also has emerged recently for MFO-catalyzed metabolism/deactivation of numerous plant allelochemicals, including compounds that induce the enzyme. MFOs are further adapted for participation in the biogenesis of substances physiologically important to insects. Moreover, the catalytic center of the MFO system, cytochrome P-450, occurs in multiple forms; the significance of this important feature is discussed.
Similar content being viewed by others
References
Abernathy, C.O., andCasida, J.E. 1973. Phyrethroid insecticides: Esterase cleavage in relation to selective toxicity.Science 179:1235–1236.
Ahmad, S. 1979. The functional roles of cytochrome P-450 mediated systems: Present knowledge and future areas of investigations.Drug. Metab. Rev. 10:1–14.
Ahmad, S. 1982. Roles of mixed-function oxidases in insect herbivory, pp. 41–47,in J.H. Visser and A.K. Minks (eds.), Proc. 5th Int. Symp. Insect-Plant Relationships. PUDOC, Wageningen.
Ahmad, S. (ed.) 1983a. Herbivorous insects: Host-Seeking Behavior and Mechanisms. Academic Press, New York, xvi + 257 pp.
Ahmad, S. 1983b. Mixed-function oxidase activity in a generalist herbivore in relation to its biology, food plants, and feeding history.Ecology 64:235–243.
Ahmad, S., andForgash, A.J. 1973. NADPH oxidation by microsomal preparations of gypsy moth larval tissues.Insect Biochem. 3:263–273.
Ahmad, S., andForgash, A.J. 1978. Gypsy moth mixed-function oxidases: Gut enzyme levels increased by rearing on a wheat germ diet.Ann. Entomol. Soc. Am. 71:449–452.
Ahmad S., Brattsten, L.B., Mullin, C.A., andYu, S.J. 1986. Enzymes involved in metabolism of plant allelochemicals,in L.B. Brattsten and S. Ahmad (eds.). Molecular Mechanisms in Insect-Plant Associations. Plenum, New York. In press.
Baldwin, I.T., andSchultz, J.C. 1983. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication betwen plants.Science 221:277–279.
Barker, R.C., Coons, L.B., Mailman, R.B., andHodgson, E. 1972. Induction of hepatic mixed-function oxidases by the insecticide mirex.Environ. Res. 5:418–424.
Beesley, S., Compton, S.G., AndJones, D. 1985. Rhodanese in insects.J. Chem. Ecol. 11:45–50.
Bell, W.J. andCardé, R.T. (eds.) 1984. Chemical Ecology of Insects. Sinauer Associates, Sunderland. xvi + 524 pp.
Berridge, M.J. 1970. A structural analysis of intestinal absorption.Symp. R. Entomol. Soc. London 5:135–151.
Blum, M.S. 1978. Biochemical defenses of insects, pp. 465–513,in M. Rockstein (ed.), Biochemistry of Insects. Academic Press, New York.
Bollenbacher, W.E., Smith, S.L., Wielgus, J.J., andGilbert, L.I. 1977. Evidence for an α-ecdysone cytochrome P-450 mixed-function oxidase in insect fat body mitochondria.Nature 268:660–663.
Boppré, M. 1978. Chemical communication, plant relationships, and mimicry in the evolution of danaid butterflies.Entomol. Exp. Appl. 24:264–277.
Bowers, W.S. 1983. Phytochemical action on insect morphogenesis, reproduction and behaviour, pp. 313–323,in D.L. Whitehead and W.S. Bowers (eds.). Natural Products for Innovative Pest Management. Pergamon Press, Oxford.
Brattsten, L.B. 1979a. Biochemical defense mechanisms in herbivores against plant allelochemicals, pp. 199–270,in G.A. Rosenthal and D.H. Janzen (eds.). Herbivores: Their interaction with Secondary Plant Metabolites Academic Press, New York.
Brattsten, L.B. 1979b. Ecological significance of mixed-function oxidations.Drug Metab. Rev. 10:35–58.
Brattsten, L.B. 1983. Cytochrome P-450 involvement in the interactions between plant terpenes and insect herbivores, pp. 173–195, in P.A. Hedin (ed.). Plant Resistance to Insects, ACS, Symposium Series Number 208. American Chemical Society, Washington, D.C.
Brattsten, L.B., andAhmad, S. (eds.) 1986. Molecular Mechanisms in Insect-Plant Associations. Plenum, New York. In press.
Brattsten, L.B., Wilkinson, C.F., andRoot, M.M. 1976. Microsomal hydroxylation of aniline in the Southern armyworm,Spodoptera eridania.Insect Biochem. 6:615–620.
Brattsten, L.B., Wilkinson, C.F., andEisner, T. 1977. Herbivore-plant interactions: Mixed-function oxidases and secondary plant substances.Science 196:1349–1352.
Brattsten, L.B., Price, S.L., andGunderson, C.A. 1980. Microsomal oxidases in midgut and fatbody tissues of a broadly herbivorous insect larva,Spodoptera eridania Cramer (Noctuidae).Comp. Biochem. Physiol. 66C: 231–237.
Crawley, M.J. 1983. Herbivory: The Dynamics of Animal-Plant Interactions. Studies in Ecology, Vol. 10. University of California Press, Berkeley, x + 437 pp.
Doane, C.C., andMcManus, M.L. (eds.) 1981. The Gypsy Moth: Research Toward Integrated Pest Management. Forest Service Tech. Bull. 1584. U.S. Dept. Agric., Washington, D.C. xix + 757 pp.
Dowd, P.F., Smith, C.M., andSparks, T.C. 1983. Detoxification of plant toxins by insects.Insect Biochem. 13:453–468.
Ehrlich, P.R., andRaven, P.H. 1964. Butterflies and plants: A study in coevolution.Evolution 18:568–608.
Ellis-Pratt, G. 1983. The mode of action of pro-allatocidins, pp. 323–355,in D.L. Whitehead and W.S. Bowers (eds.). Natural Products for Innovative Pest Management. Pergamon Press, Oxford.
Feeny, P. 1976. Plant apparency and chemical defense.Recent Adv. Phytochem. 10:1–40.
Ferkovich, S.M., Oliver, J.E., andDillard, C. 1982. Pheromone hydrolysis by cuticular and interior esterases of the antennae, legs and wings of the cabbage looper moth,Trichoplusia ni (Hubner).J. Chem. Ecol. 8:859–866.
Feyereisen, R., andDurst, F. 1978. Ecdysterone biosynthesis: A microsomal cytochrome-P-450-linked ecdysone 20-mono-oxygenase from tissues of the African migratory locust.Eur. J. Biochem. 88:37–47.
Forgash, A.J., andAhmad, S. 1974. Hydroxylation and demethylation by gut microsomes of gypsy moth larvae.Int. J. Biochem. 5:11–15.
Gould, F. 1984. Mixed function oxidases and herbivore polyphagy: The devil's advocate position.Ecol. Entomol. 9:29–34.
Gunderson, C.A.,Fleming, J.T.,and Brattsten, L.B. 1986. Biochemical defense against pulegone in southern and fall armyworm larvae.Pestic. Biochem. Physiol. In press.
Hansen, L.G., andHodgson, E. 1971. Biochemical characteristics of insect microsomes.N- andO-demethylation.Biochem. Pharmacol. 20:1569–1578.
Harborne, J.B. 1982. Introduction to Ecological Biochemistry, 2nd Ed. Academic Press, London, xvi + 278 pp.
Hough, J.A., andPimentel, D. 1978. Influence of host foliage on development survival, and fecundity of the gypsy moth.Environ. Entomol. 7:97–102.
Ivie, G.W., Bull, D.L., Beier, R.C., Pryor, N.W., andOertli, E.H. 1983 Metabolic detoxification: Mechanism of insect resistance to plant psoralens.Science 221:374–376.
Jaiswal, A.K., Gonzalez, F.J., andNebert, D.W. 1985. Human dioxin-inducible cytochrome P,-450: Complementary DNA and amino acid sequence.Science 228:80–82.
Jao, L.T., andCasida, J.E. 1974. Insect pyrethroid-hydrolyzing esterases.Pestic. Biochem. Physiol. 4:465–472.
Jones, P.B., Galeazi, D.R., Fisher, J.M., andWhitlock, Jr., J.P. 1985. Control of cytochrome P1-450 gene expression by dioxin.Science 277:1499–1502.
Kapin, M.A., andAhmad, S. 1980. Esterases in larval tissues of gypsy moth,Lymantria dispar (L.): Optimum assay conditions, quantification and characterization.Insect Biochem. 10:331–337.
Knowles, C.O., andAhmad, S. 1971. Comparative metabolism of chlorobenzilate, chloropropylate and bromopropylate acaricides by rat hepatic enzymes.Can J. Physiol. Pharmacol. 49:590–597.
Krieger, R.I., Feeny, P.P., andWilkinson, C.F. 1971. Detoxification enzymes in the guts of caterpillars: An evolutionary answer to plant defenses?Science 172:579–581.
Kreiger, R.I., Wilkinson, C.F., Hicks, L.J., andTaschenberg, E.F. 1976. Aldrin epoxidation, dihydroisodrin hydroxylation, andp-chloro-N-methyl aniline demethylation in six species of saturniid larvae.J. Econ. Entomol. 69:1–5.
Long, K.Y., andBrattsten, L.B. 1982. Is rhodanese important in the detoxification of dietary cyanide in southern armyworm (Spondoptera eridania Cramer) larvae?Insect Biochem. 12:367–375.
Marty, M.A., andKrieger, R.I. 1984. Metabolism of uscharidin, a milkweed cardenolide, by tissue homogenates of monarch butterfly,Danaus plexippus L.J. Chem. Ecol. 10:945–956.
May, M.L., andAhmad, S. 1983. Host location in Colorado potato beetle: Searching mechanisms in relation to oligophagy, pp. 173–199,in S. Ahmad (ed.). Herbivorous Insects: Host-Seeking Behavior and Mechanisms. Academic Press, New York.
Mattocks, A.R. 1972. Toxicity and metabolism ofSenecio alkaloids, pp. 179–200,in J.B. Harborne (ed.). Phytochemical Ecology. Academic Press, New York.
Miller, G.L. 1959. Protein determination for large number of samples.Anal. Chem. 31:964.
Mullin, C.A., andCroft, B.A. 1983. Host-related alterations of detoxification enzymes inTetranychus urticae (Acari: Tetranychidae).Environ. Entomol. 12:1278–1281.
Mullin, C.A., andCroft, B.A. 1984. Trans-epoxide hydrolase: A key indicator enzyme for herbivory in arthropods.Experientia 40:176–178.
Mullin, C.A., Croft, B.A., Strickler, K., Matsumura, F., andMiller, J.R. 1982. Detoxification enzyme differences between a herbivorous and predatory mite.Sciecne 217:1270–1272.
Odell, T.M., andRollinson, W.D. 1966. A technique for rearing gypsy moth,Porthetria dispar (L.).J. Econ. Entomol. 59:741–742.
Rhoades, D.F., andCates, R.G. 1976. Toward a general theory of plant antiherbivory.Recent Adv. Phytochem. 10:168–213.
Rosenthal, G.A., andJanzen, D.H. (eds.) 1979. Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York, xiii + 718 pp.
Rosenthal, G.A., Hughes, C.G., andJanzen, D.H. 1982.l-Canavanine, a dietary nitrogen source for the seed predatorCaryedes brasiliensis (Bruchidae).Science 218:353–355.
Schmidt-Nielsen, K. 1979. Animal Physiology: Adaptation to Environment, 2nd. Ed. Cambridge University Press, Cambridge, x + 560 pp.
Schultz, J.C., andBaldwin, I.T. 1982. Oak leaf quality declines in response to defoliation by gypsy moth larvae.Science 217:149–151.
Scriber, J.M. 1984. Host-plant suitability, pp. 159–202,in W.J. Bell and R.T. Carde (eds.). Chemical Ecology of Insects. Sinauer Associates, Sunderland, MA.
Scriber, J.M., andSlansky, F. 1981. The nutritional ecology of immature insects.Annu. Rev. Entomol. 26:183–211.
Scudder, G.G.E., andMeredith, J. 1982. The permeability of the midgut of three insects to cardiac glycosides.J. Insect Physiol. 28:689–694.
Sparks, T.C., andHammock, B.D. 1979. Induction and regulation of juvenile hormone esterases during the last larval instar of the cabbage looper,Trichoplusia ni.J. Insect Physiol. 25:551–560.
Thongsinthusak, T., andKrieger, R.I. 1976. Dihydroisodrin hydroxylation as an indicator of monooxygenase capability of black cutwormAgrotis ypsilon and cabbage looperTrichoplusia ni larvae.Comp. Biochem. Physiol. 54C:7–12.
Tilak, T.G., Nagarajan, V. andTupule, P.G. 1975. Microsomal metabolism as a determinant of aflatoxin toxicity.Experientia 31:935.
Trammell, D.J. 1982. In vitro metabolism of (+)-pulegone, (-)-carvone and (+)-carvone by southern armyworm (Spodoptera eridania) microsomes. MS thesis, Georgia Institute of Technology, Atlanta, August 1982.
Turunen, S., andChippendale, G.M. 1977. Ventricular esterases: Comparison of their distribution within the larval midgut of four species of Lepidoptera.Ann. Entomol. Soc. Am. 70:146–149.
Yu, S.J. 1986. Consequences of foreign compound-metabolizing enzymes in insects,in L.B. Brattsten and S. Ahmad (eds.). Molecular Mechanisms in Insect-Plant Associations. Plenum, New York. In press.
Yu, S.J., Berry, R.E., andTerriere, L.C. 1979. Host-plant stimulation of detoxifying enzymes in a phytophagous insect.Pestic. Biochem. Physiol. 12:280–284.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ahmad, S. Enzymatic adaptations of herbivorous insects and mites to phytochemicals. J Chem Ecol 12, 533–560 (1986). https://doi.org/10.1007/BF01020571
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01020571