Abstract
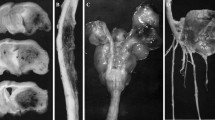
The organophosphates trichlorfon, dichlorvos, dimethoate, soman, triortho-cresyl phosphate (TOCP), and the diethoxy-analogue of trichlorfon (O,O-diethyl 2,2,2-trichloro-1-hydroxyethylphosphonate, ethyl-trichlorfon), were administrated to guinea pigs between day 42 and 46 of gestation. When the offsprings were examined at birth, there was a severe reduction in brain weight in the case of trichlorfon and dichlorvos, but not after treatment with the other organophosphates. The reduction in weight was most pronounced for cerebellum, medulla oblongata, thalamus/hypothalamus and quadrigemina. The effect was less marked for cerebral cortex and hippocampus. Since soman, a potent anticholinesterase, and TOCP, an inhibitor of neuropathy target esterase, did not show any effects, this excludes that the brain hypoplasia can be caused by inhibition of these two enzymes. Further, the lack of effect with ethyl-trichlorfon has shed some light on the part of the trichlorfon molecule which could be involved in the formation of the hypoplasia. It is suggested that alkylation of DNA may be involved in the development of the lesion. The possible consequences for a teratogenic effect of trichlorfon and dichlorvos on humans are discussed.
Similar content being viewed by others
References
Kronevi, T. 1977. Kan Neguvon®-behandling av dräktige suggor orsaka cerebellär hypoplasi hos gris? (Can Neguvon®-treatment of pregnant sows-cause cerebellar hypoplasia in piglets?) Svensk Veterinärtidning 29:931–932.
Knox, B., Askaa, J., Basse, A., Bitsch, V., Eskildsen, M., Mandrup, M., Ottosen, H. E., Øverby, E., Pedersen, K. B., and Rasmussen, F. 1978. Congenital ataxia and tremor with cerebellar hypoplasia in piglets born by sows treated with Neguvon® vet. during pregnancy. Nord. Vet. Med. 30:538–545.
Fatzer, R., Häni, H., and Scholl, E. 1981. Kongenital Tremor und zerebelläre Hypoplasie bei Ferkeln nach Behandlung der Mutterschweine mit Neguvon® während der Trächtigkeit. Schweiz Arch. Tierheilkd. 123:29–36.
Gamlem, H. N., Lund, A., Moen, J. H., and Berge, G. N. 1983. Kongenital tremor og cerebellum-hypoplasi hos spedgriser som en mulig følge av Neguvon®-behandling av drektige purker. (Congenital tremor and cerebellar hypoplasia in piglets associated with trichlorfon-treatment of pregnant sows). Norsk Veternærtidsskrift 6:385–387.
Pope, A. M., Heavner, J. E., Guarnieri, J.-A., and Knobloch, C. P. 1986. Trichlorfoninduced congenital cerebellar hypoplasia in neonatal pigs. J. Am. Vet. Med. Assoc. 189:781–783.
Berge, G. N., Fonnum, F., and Brodal, P. 1987. Neurotoxic effects of prenatal trichlorfon administration in pigs. Acta Vet. Scand. 28:321–332.
Berge, G. N., Fonnum, F., Søli, N. E., and Søgnen, E. 1987. Neurotoxicological examination of the piglet brain after prenatal and postnatal exposure to trichlorfon. Acta Vet. Scand. 28:313–320.
Berge, G. N., Nafstad, I., and Fonnum, F. 1986. Prenatal effects of trichlorfon on the guinea-pig brain. Arch. Toxicol. 59:30–35.
Hjelde, T., Mehl, A., Schanke, T. M., and Fonnum, F: Prenatal effects of trichlorfon on the development of different parts of the guinea-pig brain (in preparation).
Czeizel, A. E., Elek, C., Gundy, S., Métneki, J., Nemes, E., Reis, A., Sperling, K., Timár, L., Tusnády, G., and Virágh, Z. 1993. Environmental trichlorfon and cluster of congenital abnormalities. The Lancet. 341:539–542.
Nordgren, I., Bergström, M., Holmstedt, B., and Sandoz, M. 1978. Transformation and action of metrifonat. Arch. Toxicol. 41:31–41.
Barthel, W. F., Giang, P. A., and Hall, S. A. 1954. Dialkyl α-hydrocyphosphonates derived from chloral. J. Am. Chem. Soc. 76:4186–87.
De Roos, A. M., and Toet, H. J. 1959. The preparation of some iso-propyl p-nitrophenyl alkylphosphonates. Rec. Trav. Chim. 78:59.
Barrett, D. S., and Oehme, F. W. 1984. A review of organophosphoros ester-induced delayed neurotoxicity. Vet. Hum. Toxicol. 27:22–37.
Blair, D., Hoadley, E. C., and Hutson, D. H. 1975. The distribution of dichlorvos in the tissues of mammals after it's inhalation or intravenous administration. Toxicol. Appl. Pharmacol. 31:243–253.
Sterri, S. H., Lyngaas, S., and Fonnum, F. 1981. Toxicity of soman after repetitive injection of sublethal doses in guinea-pig and mouse. Acta Pharmacol. Toxicol. 49:8–13.
Fonnum, F. 1975. A rapid radiochemical method for the determination of choline acetyltransferase. J. Neurochem. 25:407–409.
Fonnum, F., Walaas, I., and Iverseh, E. G. 1977. Localization of GABAergic, cholinergic and aminergic structures on the mesolimbic system. J. Neurochem. 29:221–230.
Ellman, G. L., Courtney, K. D., Andres, V. Jr., and Featherstone, R. M. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88–95.
Harbison, R. D., Olubadewo, J., Dwivedi, C., and Sastry, B. V. R. 1975. Proposed role of the placental cholinergic system in the regulation of fetal growth and development, p.p 107–120 in P. L. Morselli, S. Garattini, and F. Sereni, eds. In: Basic and Therapeutic Aspects of Perinatal Pharmacology, Raven Press, New York.
Coult, D. B., Marsh, D. J., and Read, G. 1966. Dealkylation studies on inhibited acetylcholinesterase. Biochem. J. 98:869–873.
Abou-Donia, M. B. 1981. Organophosphorus ester-induced delayed neurotoxicity. Ann. Rev. Pharmacol. Toxicol. 21:511–548.
Johnson, M. K. 1981. Delayed neurotoxicity-Do trichlorfon and/or dichlorvos cause delayed neuropathy in man or in test animals? Acta Pharmacol. Toxicol. 49, suppl. V, 87–98.
Braun, R., Schöneich, J., Weissflog, L., and Dedek, W. 1982. Activity of organophosphorous insecticides in bacterial tests for mutagenicity and DNA repair-direct alkylation vs. metabolic activation and breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos, demethyl dichlorvos and demethyl vinylbutonate. Chem. Biol. Interactions. 39:339–350.
Dedek, W. 1981. Guanine N7-alkylation in mice in vivo by metrifonate-discussion of possible genotoxic risk in mammals. Acta Pharmacol. Toxicol. 49, suppl. V, 7–14.
Hofer, W. 1981. Chemistry of metrifonat and dichlorvos. Acta Pharmacol. Toxicol. 49, suppl. V, 7–14.
Holmstedt, B., Nordgren, I., Sandoz, M., and Sundwall, A. 1978. Metrifonate. Summary of toxicological and pharmacological information available. Arch. Toxicol. 41:3–29.
Bedford, C. T., and Robinson, J. 1972. The alkylating properties of organophosphates. Xenobiotica 2:307–337.
Wooder, M. F., and Wright, A. S. 1981. Alkylation of DNA by organophosphorous pesticides. Acta Pharmacol. Toxicol. 49: suppl. V, 51–55.
Wild, D. 1975. Mutagenicity studies on organophosphorous insecticides. Mutat. Res. 32:133–150.
Krueger, H. R., O'Brien, R. D., and Dauterman, W. C. 1960. Relationship between metabolism and differential toxicity in insects and mice of diazinon, dimethoate, parathion and acethion. J. Econ. Entomol. 53:25–31.
Khera, K. S., Whalen, C., Trivett, G., and Angers, G. 1979. Teratogenicity studies on pesticidal formulations of dimethoate, diuran and lindane in rats. Bull. Environ. Contam. Toxicol. 22:522–529.
Courtney, K. D., Andrews, J. E., Springer, J., and Dalley, L. 1985. Teratogenic evaluation of the pesticides Baygon, carbofuran, dimethoate and EPN. J. Environ. Sci. Health 20:373–406.
Crebelli, R., Conti, G., Conti, L., and Carete, A. 1985. Mutagenicity of trichloroethylene, trichloroethanol and chloral hydrate inAspergillus nidulans. Mutat. Res. 155:105–111.
Russo, A., Pacchierotti, F., and Metalli, P. 1984. Nondisjunction induced in mouse spermatogenesis by chloral hydrate, a metabolite of trichloroethylene. Environ. Mutagen. 6:695–703.
Lee, G. M., Diguiseppi, J., Gawdi, G. M., and Herman, B. 1987. Chloral hydrate disrupts mitosis by increasing intracellular free calcium. J. Cell Science 88:603–612.
Löfroth, G. 1978. The mutagenicity of dichloroacetaldehyde. Z. Natufrosch. 33c:783–785.
Dobbing, J., and Sands, J. 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17:115–123.
Author information
Authors and Affiliations
Additional information
Special issue dedicated to Dr. Bernard W. Agranoff.
Rights and permissions
About this article
Cite this article
Mehl, A., Schanke, T.M., Johnsen, B.A. et al. The effect of trichlorfon and other organophosphates on prenatal brain development in the guinea pig. Neurochem Res 19, 569–574 (1994). https://doi.org/10.1007/BF00971332
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00971332