Abstract
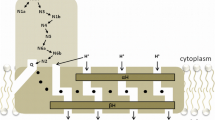
The proton-translocating NADH: ubiquinone oxidoreductase (complex I) is a large, multi-subunit and multi-redox centre enzyme which is found in the mitochondrial inner membrane and plasma membrane of some bacteria. In this minireview an attempt has been made to critically discuss selected topics in the structure and function of this enzyme. A special emphasis is given to the iron-sulphur cluster and to the proteins that may bind them. Previous suggestions for the mechanism of proton-translocation by complex I are discussed. Subcomplexes that contain several but not all of the subunits of the intact mitochrondrial enzyme are described and analysed in order to identify the functional core of the enzyme. The data on the trans-membrane organisation of several subunits is examined. It is hoped that most of the suggestions as well as the questions raised here could be experimentally tested in the near future.
Similar content being viewed by others
References
Albracht, S. P. J., Dooijewaard, G., Leeuwerik, F. J., and Van Swol, B. (1977).Biochim. Biophys. Acta 459, 300–317.
Albracht, S. P. J., Van Verseweld, H. W., Hagen, W. R., and Kalkman, M. L. (1980).Biochim. Biophys. Acta 593, 173–186.
Beinert, H. and Albracht, S. P. J. (1982)Biochim. Biophys. Acta 683, 245–277.
Brown, G. C., and Brand, M. D. (1988).Biochem. J. 252, 473–479.
Chen, S., and Guillory, R. T. (1981).J. Biol. Chem. 256, 8318–8323.
Davidson, E., Ohnishi, T., Atta-Asafo-Adjei, E., and Daldal, F. (1992).Biochemistry 31, 3342–3351.
De Vries, S., and Marres, C. A. M. (1987).Biochim. Biophys. Acta 895, 205–239.
Dupuis, A., Skehel, M. J. and Walker, J. E. (1991).Biochemistry 30, 2954–2960.
Fearnley, I. M. and Walker, J. E. (1992).Biochim. Biophys. Acta 1140, 105–134.
Fearnley, I. M., Runswick, M. J., and Walker, J. E. (1989).EMBO J. 8, 665–672.
Finel, M., and Wikström, M. (1986).Biochim. Biophys. Acta. 851, 99–108.
Finel, M., Skehel, J. M., Albracht, S. P. J., Fearnley, I. M., and Walker, J. E. (1992).Biochemistry 31, 11425–11434.
Freidrich, T., Hofhaus, G., Ise, W., Nehls, U., Schmitz, B., and Weiss, H. (1989).Eur. J. Biochem. 180, 173–180.
Galante, Y. M., and Hatefi, Y. (1979).Arch. Biochem. Biophys. 192, 559–568.
Han, A-L., Yagi, T. and Hatefi, Y. (1989).Arch. Biochem. Biophys. 275, 166–173.
Hatefi, Y., and Hanstein, W. G. (1973).Biochemistry 12, 3515–3522.
Hofhaus, G., Weiss, H., and Leonard, K. (1991).J. Mol. Biol. 221, 1027–1043.
Ingledew, W. J., and Ohnishi, T. (1980).Biochem. J. 186, 111–117.
Jaworowski, A., Mayo, G., Shaw, D. C., Capbell, H. D., and Young, I. G. (1981).Biochemistry 20, 3621–3628.
Kotlyar, A. B., Sled, V. D., Burbaev, D. Sh., Moroz, I. A., and Vinogradov, A. D. (1990).FEBS Lett. 264, 17–20.
Kowal, A. T., Morningstar, J. E., Johnson, M. K., Ramsey, R. R., and Singer, T. P. (1986).J. Biol. Chem. 261, 9239–9245.
Krishnamoorthy, G., and Hinkle, P. C. (1988).J. Biol. Chem. 263, 17566–17575.
Leonard, K., Haikar, H., and Weiss, H. (1987).J. Mol. Biol. 194, 277–286.
Masui, R., Wakabayashi, S., Matsubara, H., and Hatefi, Y. (1991).J. Biochem. 109, 534–543.
Meinhardt, S. W., Kula, T., Yagi, T., Lillich, T., and Ohnishi, T. (1987).J. Biol. Chem. 262, 9147–9153.
Mitchell, P. (1961).Nature (London) 191, 144–148.
Nehls, U., Friedrich, T., Schmiede, A., Ohnishi, T., and Weiss, H. (1992).J. Mol. Biol. 227, 1032–1042.
Ohnishi, T. (1979). InMembrane Proteins in Energy Transduction (Capaldi, R. A., ed.), Marcel Dekker, New York, pp 1–87.
Ohnishi, T. (1987).Curr. Top. Bioenerg. 15, 37–65.
Ohnishi, T., and Salerno, J. C. (1982). InIron-Sulfur Proteins Vol. 4 (Spiro, T. G., ed.), Wiley, New York, pp. 285–327.
Ohnishi, T., Ragan, C. I., and Hatefi, Y. (1985).J. Biol. Chem. 260, 2782–2788.
Orme-Johnson, N. R., Hansen, R. E., and Beinert, H. (1974).J. Biol. Chem. 249, 1922–1927.
Paech, C., Reynolds, J. G., Singer, T. P., and Holm, R. H. (1981).J. Biol. Chem. 256, 3167–3170.
Paech, C., Friend, A., and Singer, T. P. (1982).Biochem. J. 203, 244–481.
Pagani, S., and Galante, Y. M. (1983).Biochim. Biophys. Acta 742, 278–284.
Patel, S. D., Cleeter, M. W. J., and Ragan, C. I. (1988).Biochem. J. 256, 529–535.
Pilkington, S. J., and Walker, J. E. (1989).Biochemistry 28, 3257–3264.
Pilkington, S. J., Skehel, J. M., Gennis, R. B., and Walker, J. E. (1991a).Biochemistry 30, 2166–2175.
Pilkington, S. J., Skehel, J. M. and Walker, J. E. (1991b).Biochemistry 30, 1901–1098.
Ragan, C. I. (1987).Curr. Top. Bioenerg. 15, 1–36.
Ragan, C. I., and Hinkle, P. C. (1975).J. Biol. Chem. 250, 8472–8476.
Ragan, C. I., Ohnishi, T., and Hatefi, Y. (1986). InFrontiers of Ironsulfur Protein Research (Matsubara, H.,et al., eds.), Japan Scientific Societies Press, Tokyo, pp 220–231.
Runswick, M. J., Gennis, R. B., Fearnley, I. M., and Walker, J. E. (1989).Biochemistry 28, 9452–9459.
Salerno, J. C., Ohnishi, T., Lim, J., Widger, W. R., and King, T. E. (1977).Biochem. Biophys. Res. Commun. 75, 618–624.
Schmidt, M., Friedrich, T., Wallrath, J., Ohnishi, T., and Weiss, H. (1992).FEBS Lett. 313, 8–11.
Schneider, K., Schlegel, H. G., Cammack, R., and Hall, D. O. (1979).Biochim. Biophys. Acta 578, 445–461.
Schneider, K., Cammack, R., and Schlegel, H. G. (1984).Eur. J. Biochem. 142, 75–84.
Tran-Betcke, A., Warnecke, U., Böcker, C., Zabarosch, C., and Friedrich, B. (1990).J. Bacteriol. 172, 2920–2929.
Van Belzen, R. (1991). Ph.D. Thesis, University of Amsterdam.
Van Belzen, R., and Albracht, S. P. J. (1989).Biochem. Biophys. Acta 974, 311–320.
Van Belzen, R., Van Gaalen, M. C. M., Cuypers, P. A., and Albracht, S. P. J. (1990).Biochim. Biophys. Acta 1017, 152–159.
Van Belzen, R., De Jong, A. M. Ph., and Albracht, S. P. J. (1992).Eur. J. Biochem. 209, 1019–1022.
Walker, J. E. (1992).Q. Rev. Biophys.,25, 253–324.
Wang, D-C., Meinhardt, S. W., Sackmann, U., Weiss, H., and Ohnishi, T. (1991).Eur. J. Biochem. 197, 257–264.
Weiss, H., Friedrich, T., Hofhaus, G., and Preis, D. (1991).Eur. J. Biochem. 197, 563–576.
Wikström, M. (1984).FEBS Lett. 169, 300–304.
Wikström, M., Krab, K., and Saraste, M. (1981).Annu. Rev. Biochem. 50, 623–655.
Xu, X., Matsuno-Yagi, A., and Yagi, T. (1991).Biochemistry 30, 8678–8684.
Yagi, T. (1991).J. Bioenerg. Biomembr. 23, 211–225.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Finel, M. The proton-translocating NADH: Ubiquinone oxidoreductase: A discussion of selected topics. J Bioenerg Biomembr 25, 357–366 (1993). https://doi.org/10.1007/BF00762461
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00762461