Abstract
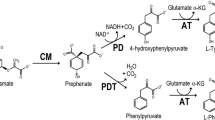
The reaction catalyzed by chorismate mutase (EC 5.4.99.5) is a crucial step for biosynthesis of two aromatic amino acids as well as for the synthesis of phenylpropanoid compounds. The regulatory properties of two chorismate-mutase isoenzymes expressed in Nicotiana silvestris Speg. et Comes are consistent with their differential roles in pathway flow routes ending with l-phenylalanine and l-tyrosine on one hand (isoenzyme CM-1), and ending with secondary metabolites on the other hand (isoenzyme CM-2). Isoenzyme CM-1 was very sensitive to allosteric control by all three aromatic amino acids. At pH 6.1, l-tryptophan was a potent allosteric activator (K a =1.5 μM), while feedback inhibition was effected by l-tyrosine (K i =15 μM) or by l-phenylalanine (Ki=15 μM). At pH 6.1, all three effectors acted competitively, influencing the apparent K m for chorismate. All three allosteric effectors protected isoenzyme CM-1 at pH 6.1 from thermal inactivation at 52° C. l-Tryptophan abolished the weak positive cooperativity of substrate binding found with isoenzyme CM-1 only at low pH. At pH 7.2, the allosteric effects of l-tyrosine and l-tryptophan were only modestly different, in striking contrast to results obtained with l-phenylalanine. At pH 7.2 (i) the K i for l-phenylalanine was elevated over 30-fold to 500 μM, (ii) the kinetics of inhibition became non-competitive, and (iii) l-phenylalanine now failed to protect isoenzyme CM-1 against thermal inactivation. l-Phenylalanine may act at different binding sites depending upon the intracellular pH milieu. In-vitro data indicated that the relative ability of allosteric activation to dominate over allosteric inhibition increases markedly with both pH and temperature. The second isoenzyme, CM-2, was inhibited competitively by caffeic acid (K i =0.2 mM). Aromatic amino acids failed to affect CM-2 activity over a broad range of pH and temperature. Inhibition curves obtained in the presence of caffeic acid were sigmoid, yielding an interaction coefficient (from Hill plots) of n′=1.8.
Similar content being viewed by others
Abbreviations
- DAHP synthase:
-
3-deoxy-d-arabino-heptulosonate 7-phosphate synthase
References
Arfin, S.M., Koziell, D.A. (1973) Acetolactate synthase of Pseudomonas aeruginosa. Biochim. Biophys. Acta 321, 348–355
Bickel, H., Palme, L., Schultz, G. (1978) Incorporation of shikimate and other precursors into aromatic amino acids and prenylquinones of isolated spinach chloroplasts. Phytochemistry 17, 119–124
Byng, G.S., Jensen, R.A. (1983) Impact of isozymes upon partitioning of carbon flow and regulation of aromatic biosynthesis in prokaryotes. In: Isozymes, vol. 8, pp. 115–140, Ratazzi, M.C., Scandalios, J.G., Whitt, G.S., eds. Liss, New York
Cotton, R.G., Gibson, F. (1965) The biosynthesis of phenylalanine and tyrosine: enzymes converting chorismic acid into prephenic acid and their relationship to prephenate dehydratase and prephenate dehydrogenase. Biochim. Biophys. Acta 100, 76–78
d'Amato T.A., Ganson, R.J., Gaines, C.G., Jensen, R.A. (1984) Subcellular localization of chorismate-mutase isoenzymes in protoplasts from mesophyll and suspension-cultured cells of Nicotiana silvestris. Planta 162, 104–108
Gaines, C.G., Byng, G.S., Whitaker, R.J., Jensen, R.A. (1982) l-Tyrosine regulation and biosynthesis via arogenate dehydrogenase in suspension-cultured cells of Nicotiana silvestris Speg. et Comes. Planta 156, 233–240
gilchrist, D.G., Kosuge, T. (1974) Regulation of aromatic amino acid biosynthesis in higher plants. Properties of an aromatic amino acid-sensitive chorismate mutase (CM-1) from mung bean. Arch. Biochem. Biophys 164, 95–105
gilchrist, D.G., Kosuge, T. (1980) Aromatic amino acid biosynthesis and its regulation. In: The biochemistry of plants, pp. 507–531, Miflin, B.J., ed. Academic Press, New York London
Goers, S.K., Jensen, R.A. (1984) Separation and characterization of two chorismate-mutase isozymes from Nicotiana silvestris. Planta 162, 109–116
Gottlieb, L.D. (1982) Conversion and duplication of isozymes in plants. Science 216, 373–380
Graziana, A., Boudet, A.M. (1980) 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase from Zea mays: general properties and regulation by tryptophan. Plant Cell Physiol. 21, 793–802
Jensen, R.A., Nester, E.W. (1966) Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. II. The enzymology of feedback inhibition of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthetase. J. Biol. Chem. 241, 3373–3380
Jensen, R.A. (1970) Taxonomic implications of temperature dependence of the allosteric inhibition of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthetase in Bacillus. J. Bacteriol. 120, 489–497
Nishioka, L., Woodin, t. (1972) Improved assay for phenylpyruvic acid. Anal. Biochem. 45, 617–623
Reinink, M., Borstlap, A.C. (1982) 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase from pea leaves: inhibition by l-tyrosine. Plant Sci. Lett. 26, 167–171
Rubin, J.L., Gaines, C.G., Jensen, R.A. (1982) Enzymological basis for herbicidal action of glyphosate. Plant Physiol. 70, 833–839
Woodin, T.S., Nishioka, L., Hsu, A. (1978) Comparison of chorismate mutase isozyme patterns in selected plants. Plant Physiol 61, 949–952
Woodin, T.S., Nishioka, L. (1973) Evidence for three isozymes of chorismate mutase in alfalta. Biochim. Biophys. Acta 309, 211–223
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goers, S.K., Jensen, R.A. The differential allosteric regulation of two chorismate-mutase isoenzymes of Nicotiana silvestris . Planta 162, 117–124 (1984). https://doi.org/10.1007/BF00410207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00410207