Abstract
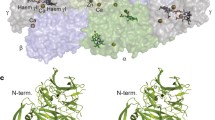
The hydroxylamine oxidoreductase from Nitrosomonas europaea was prepared to apparent electrophoretic homogeneity. Electron microscopy of negatively stained preparations of the sample revealed an overall diameter of about 8.8 nm of the enzyme particle. The native structure was determined as a tetrahedron-like assembly of identical subunits exhibiting four protein masses.
Similar content being viewed by others
Abbreviations
- ESI :
-
Electron spectroscopic imaging
- HAO :
-
Hydroxylamine oxidoreductase
References
Anderson KK, Kent TA, Lipscomb JD, Hooper AB, Münck E (1984) Mössbauer, EPR, and optical studies of the P-460 center of hydroxylamine oxidoreductase from Nitrosomonas. J Biol Chem 259:6833–6840
Arciero DM, Hooper AB (1993) Hydroxylamine oxidoreductase from Nitrosomonaseuropaea is a multimer of an octa-heme subunit. J Biol Chem 268:14645–14654
Beuscher N, Mayer F, Gottschalk G (1974) Citrate lyase of Rhodopseudomonas gelatinosa: purification, electron microscopy and subunit structure. Arch Microbiol 100:307–328
Fuchs J, Johannssen W, Rohde M, Mayer F (1988) Pyruvate carboxylase from Pseudomonas citronellolis: shape of the enzyme; and localization of its prosthetic biotin group by electron microscopic affinity labeling. FEBS Lett 231:102–106
Gerberding H, Norris B, Miller DJ, Mayer F (1991) Tetrameric native structure of the peridinin-chlorophyll a-binding protein from Symbiodinium sp. J Plant Physiol 137:285–290
Hooper AB, Nason A (1965) Characterization of hydroxylamine-cytochrome c reductase from the chemoautotrophs Nitrosomonas europaea and Nitrosocystis oceanus. J Biol Chem 240:4044–4057
Johannssen W, Gerberding H, Rohde M, Zaborosch C, Mayer F (1991) Structural aspects of the soluble NAD-dependent hydrogenase isolated from Alcaligenes eutrophus H16 and from Nocardia opaca 1b. Arch Microbiol 155:303–308
Jovin T, Chrambach A, Naughton MA (1964) An apparatus for preparative temperature-regulative polyacrylamide gel electrophoresis. Anal Biochem 9:351–359
Markham R, Frey S, Hills GJ (1963) Methods for the enhancement of image detail and accentuation of structure in electron microscopy. Virology 20:88–102
Masson P, Arciero DM, Hooper AB, Balny C (1990) Electrophoresis at elevated hydrostatic pressure of the multiheme hydroxylamine oxidoreductase. Electrophoresis 11:128–133
Mayer F, Wallace JC, Keech DB (1980) Further electron microscope studies on pyruvate carboxylase. Eur J Biochem 112: 265–272
Mayer F, Elliot JI, Sherod D, Ljungdahl LG (1982) Formyltetrahydrofolate synthase from Clostridium thermoaceticum. An electron microscopic study and specific interaction of the enzyme with ATP and ADP. Eur J Biochem 124:397–404
Miller DJ, Wood PM (1982) Characterization of the c-type cytochromes of Nitrosomonas europaea with the aid of fluorescent gels. Biochem J 207:511–517
Ottensmeyer FP, Andrew JW (1980) High-resolution microanalysis of biological specimens by electron energy loss spectroscopy and by electron spectroscopic imaging. J Ultrastruct Res 72:336–348
Rohde M, Lim F, Wallace JC (1986) Pyruvate carboxylase from Saccharomyces cerevisiae. Quaternary structure, effects of allosteric ligands and binding of avidin. Eur J Biochem 156: 15–22
Sayavedra-Soto LA, Hommes NG, Arp DJ (1994) Characterization of the gene encoding hydroxylamine oxidoreductase in Nitrosomonas europaea. J Bacteriol 176:504–510
Specka U, Mayer F (1993) Cellular location, activity states, and macromolecular organization of glucoamylase in Clostridium thermosaccharolyticum. Arch Microbiol 160:284–287
Terry KR, Hooper AB (1981) Hydroxylamine oxidoreductase: a 20-heme, 200,000 molecular weight cytochrome c with unusual denaturation properties which forms a 63,000 molecular weight monomer after heme removal. Biochemistry 20:7026–7032
Valentine RC, Shapiro BM, Stadtman ER (1968) Regulation of glutamine synthetase. 12. Electron microscopy of the enzyme from Escherichia coli. Biochemistry 7:2143–2152
Wackett LP, Hartwieg EA, King JA, Orme-Johnson WH, Walsh CT (1987) Electron microscopy of nickel-containing methanogenic enzymes: methylreductase and F420-reducing hydrogenase. J Bacteriol 169:718–727
Yamanaka T, Shinra M (1974) Cytochrome c-552 and cytochrome c-554 derived from Nitrosomonas europaea. Purification, properties and their function in hydroxylamine oxidation. J Biochem 75:1265–1273
Yamanaka T, Shinra M, Takahashi K, Shibasaka M (1979) Highly purified hydroxylamine oxidoreductase derived from Nitrosomonas europaea. J Biochem 86:1101–1108
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoppert, M., Mahony, T.J., Mayer, F. et al. Quaternary structure of the hydroxylamine oxidoreductase from Nitrosomonas europaea . Arch. Microbiol. 163, 300–306 (1995). https://doi.org/10.1007/BF00393384
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393384