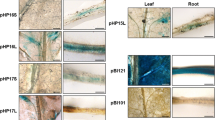
Summary
The thermoregulated expression of the soybean heat shock (hs) gene Gmhsp17.3-B is regulated via the heat shock promoter elements (HSEs), but full promoter activity requires additional sequences located upstream of the HSE-containing region. Structural features within this putative enhancer region include a run of simple sequences which are also present upstream of HSE-like sequences of other soybean hs genes, and three perfect CCAAT box sequences located immediately upstream from the most distal HSE of the promoter. A series of heterologous and homologous promoter fusions linked to the chloramphenicol acetyl transferase (CAT) gene was constructed and examined in transgenic tobacco plants. The region containing the AT-rich domain of the 5′ flanking region was unable to direct transcription from the TATA box of a truncated ΔCaMV35S promoter. Heat-inducible CAT activity was detectable when additional sequences from the native promoter containing three CCAAT boxes and a single HSE were present in the constructions. Complete reconstitution of the native hs promoter/enhancer region increased hs specific CAT activities only very little, but deletion of CCAAT box sequences reduced CAT expression fivefold. Our results suggest that AT-rich sequences have a moderate effect on thermoinducible expression levels of the soybean heat shock gene and that CCAAT box sequences act cooporatively with HSEs to increase the hs promoter activity.
Similar content being viewed by others
References
Amin J, Ananthan J, Voellmy R (1988) Key features of heat shock regulatory elements. Mol Cell Biol 8:3761–3769
Arnold W, Pühler A (1988) A family of high-copy-number plasmid vectors with single end-label sites for rapid nucleotide sequencing. Gene 70:171–179
Baumann G, Raschke E, Bevan M, Schöffl F (1987) Functional analysis of sequences required for transcriptional activation of a soybean heat shock gene in transgenic tobacco plants. EMBO J 6:1161–1166
Bevan MW (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
Bienz M (1986) A CCAAT box confers cell-type-specific regulation on the Xenopus hsp70 gene in oocytes. Cell 46:1037–1042
Bienz M, Pelham HRB (1986) Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell 45:753–760
Bienz M, Pelham HRB (1987) Mechanisms of heat shock gene activation in higher eukaryotes. Adv Genet 24:31–72
Clos J, Westwood JT, Becker PB, Wolson S, Lambert K, Wu C (1990) Molecular cloning and expression of a hexameric Drosophila heat shock factor subject to negative regulation. Cell 63:1085–1097
Czarnecka E, Key JL, Gurley WB (1989) Regulatory domains of the Gm hsp17.5-E heat shock gene promoter of soybean. A mutational analysis. Mol Cell Biol 9:3457–3463
Czarnecka E, Fox PC, Gurley WB (1990) In vitro interaction of nuclear proteins with the promoter of soybean heat shock gene Gmhsp17.3-E. Plant Physiol 94:935–943
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version 11. Plant Mol Biol Rep 1:19–21
Gurley WB, Czarnecka E, Nagao RT, Key JL (1986) Upstream sequences required for efficient expression of a soybean heat shock gene. Mol Cell Biol 6:559–565
Helm KW, Abernethy RH (1990) Heat shock proteins and their mRNAs in dry and early imbibing embryos of wheat. Plant Physiol 93:1626–1633
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Jakobsen BK, Pelham HRB (1991) A conserved heptapeptide restrains the activity of yeast heat shock transcription factor. EMBO J 10:369–375
Jensen EQ, Marcker KA, Schell J, de Bruijn FJ (1988) Interaction of nodule specific, trans-acting factor with distinct DNA elements in the soybean leghaemoglobin lbc 3 5′ upstream region. EMBO J 7:1265–1271
Jofuku KD, Okamuro JK, Goldberg RB (1987) Interaction of an embryo DNA binding protein with a soybean lectin gene upstream region. Nature 328:734–737
Key JL, Lin CY, Chen YM (1981) Heat shock proteins of higher plants. Proc Natl Acad Sci USA 78:3526–3530
Kingston RE, Schütz TJ, Larin Z (1987) Heat-inducible human factor that binds to a human hsp70 promoter. Mol Cell Biol 7:1530–1534
Morgan WD, Williams GT, Morimoto RJ, Greene J, Kingston RE, Tjian R (1987) Two transcriptional activators, CCAATbox-binding transcription factor and heat shock transcription factor, interact with a human hsp70 gene promoter. Mol Cell Biol 7:1129–1138
Morimoto RI, Tissières A, Georgopoulos C (1990) Stress Proteins in Biology and Medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Neumann D, Never L, Parthier B, Rieger R, Scharf KD, Wollgiehn R, zur Nieden U (1989) Heat shock and other stress response systems of plants. Biol Zentbl 108:1–156
Nover L (1987) Expression of heat shock genes in homologous and heterologous systems. Enzyme Microbiol Technol 9:130–144
Parker CS, Topol J (1984) A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp70 gene. Cell 37:273–283
Pedersen TJ, Arwood LJ, Spiker S, Guiltinan MJ, Thompson F (1991) High mobility group chromosomal proteins bind to ATrich tracts flanking plant genes. Plant Mol Biol 16:95–104
Pelham HRB (1982) A regulatory upstream promoter element in the Drosophila hsp70 heat shock gene. Cell 30:517–528
Perisic O, Xiao H, Lis JT (1989) Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 by recognition unit. Cell 59:797–806
Raschke E, Baumann G, Schöffl F (1988) Nucleotide sequence analysis of soybean small heat shock protein genes belonging to two different multigene families. J Mol Biol 199:549–557
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Scharf KD, Rose S, Zott W, Schoffl F, Never L (1990) Three tomato genes code for heat stress transcription factors with a region of remarkable homology to the DNA-binding domain of the yeast HSF. EMBO J 9:4495–4501
Schöffl F, Baumann G (1985) Thermo-induced transcripts of a soybean heat shock gene after transfer into sunflower using a Ti plasmid vector. EMBO J 4:1119–1124
Schöffl F, Baumann G, Raschke E, Bevan MW (1986) The expression of heat shock genes in higher plants. Philos Trans R Soc Lend [Biol] 314:453–468
Schöffl F, Baumann G, Raschke E (1988) The expression of heat shock genes. A model for environmental stress response. In: Verma DPS, Goldberg RB (eds) Plant Gene Research — Temporal and Spatial Regulation of Plant Genes. Springer Verlag, Wien New York, pp 253–273
Schöffl F, Rieping M, Baumann G, Bevan MW, Angermüller S (1989) The function of plant heat shock promoter elements in the regulated expression of chimaeric genes in transgenic tobacco. Mol Gen Genet 217:246–253
Schöffl F, Rieping M, Raschke E (1990) Functional analysis of sequences regulating the expression of heat shock genes in transgenic plants. In: Lycett GW, Grierson D (eds) Genetic Engineering of Crop Plants. Butterworth, London pp 79–94
Schöffl F, Rieping M, Severin K (1991a) The induction of the heat shock response: activation and expression of chimaeric heat shock genes in transgenic plants. In: Herrmann RG (ed) Plant Molecular Biology. NATO-ASI Series. Plenum Press, New York, pp 685–694
Schöffl F, Diedring V, Kliem M, Rieping M, Schröder G, Severin K (1991 b) The heat shock response in transgenic plants: the use of chimaeric heat shock genes. In: Wray JL (ed) Biochemistry and molecular biology of inducible enzymes and proteins in higher plants. Cambridge University Press, Cambridge, in press
Sorger PK, Lewis MJ, Pelham HRB (1987) Heat shock factor is regulated differently in yeast and HeLa cells. Nature 329:81–84
Verwoerd TC, Dekker BMM, Hockema A (1989) A small-scale procedure for the rapid isolation of plant RNA. Nucleic Acids Res 17:2362
Vieira J, Messing J (1982) The pUC plasmid, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Vierling E, Sun A (1987) Developmental expression of heat shock proteins in higher plants. In: Cerry J (ed) Environmental Stress in Plants. Biochemical and Physiological Mechanisms associated with Environmental Stress Tolerance in Plants. Springer Verlag, New York, pp 343–354
Williams GT, Morimoto RJ (1990) Maximal stress-induced transcription from the human hsp70 promoter requires interactions with the basal promoter elements independent of rotational alignment. Mot Cell Biol 10:3125–3136
Wing D, Koncz C, Schell J (1989) Conserved function in Nicotiana tabacum of a single Drosophila hsp70 promoter heat shock element when fused to a minimal T-DNA promoter. Mot Gen Genet 219:9–16
Wu BJ, Kingston RE, Morimoto RJ (1986) Human hsp70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci USA 83:629–633
Wu C (1984) Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature 311:81–84
Xiao H, Lis JT (1988) Germline transformation used to define key features of heat shock response elements. Science 239:1139–1142
Xiao H, Perisic O, Lis JT (1991) Cooperative binding of Drosophila heat shock factor to arrays of a conserved 5 bp unit. Cell 64:585–593
Zimarino V, Wu C (1987) Induction of sequence-specific binding of Drosophila heat shock activator protein without protein syn thesis. Nature 327:727–730
Author information
Authors and Affiliations
Additional information
Communicated by H. Saedler
Rights and permissions
About this article
Cite this article
Rieping, M., Schöffl, F. Synergistic effect of upstream sequences, CCAAT box elements, and HSE sequences for enhanced expression of chimaeric heat shock genes in transgenic tobacco. Molec. Gen. Genet. 231, 226–232 (1992). https://doi.org/10.1007/BF00279795
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00279795