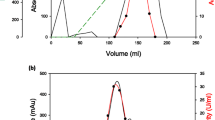
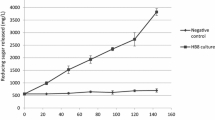
Summary
High concentrations of amylases and pullulanases were formed by continuous cultivation of Thermoanaerobacter finnii, Thermobacteroides acetoethylicus, Thermoanaerobacter ethanolicus and Clostridium thermosaccharolyticum in chemostats under starch limitation. 70% to 98% of these enzymes were transported and released into the culture fluid. These extracellular enzymes were extremely thermostable under aerobic conditions and in the absence of substrate and metal ions. The amylases and pullulanases from the first three organisms had an optimal temperature of 90°C. The enzymes from C. thermosaccharolyticum were most active at 75°C. The pH optima of the amylolytic enzymes from the microorganisms investigated ranged between 5 and 6. The addition of calcium ions in vitro significantly enhanced pullulanase activity from T. finnii and C. thermosaccharolyticum. The influence of other metal ions and cyclodextrins on the activities of the amylolytic enzymes is also described.
Similar content being viewed by others
References
Antranikian G, Gottschalk G (1987) Production of thermostable enzymes for starch hydrolysis. 4th European Congress of Biotechnology. Amsterdam, The Netherlands, pp 1–4
Antranikian G, Gottschalk G (1986) German Patent No. P 3 639267.7
Antranikian G, Zablowski, Gottschalk G (1987) Conditions for the overproduction and excretion of thermostable glucoamylase and pullulanase from Clostridium thermohydrosulfuricum DSM 567. Appl Microbiol Biotechnol 27:75–81
Antranikian G, Herzberg C, Gottschalk G (1987a) Production of thermostable α-amylase, pullulanase and α-glucosidase in continuous culture by a new Clostridium isolate. Appl Environ Microbiol, 53:1668–1673
Antranikian G, Herzberg C, Mayer F, Gottschalk G (1987b) Changes in cell-envelope structure of Clostridium strain EM 1 during massive production of amylase and pullulanase. FEMS Lett 41:193–197
Bender H, Wallenfels K (1966) Pullulanase (an amylopectin and glycogen debranching enzyme) from Aerobacter aerogenes. In: Neufeld EF, Ginsburg (eds) Method in enzymology VIII. Academic Press, New York, pp 555–562
Bergmeyer HU, Gawehn K, Grabe M (1974) Enzyme als biochemische Reagentien. In: Bergmeyer HU (ed) Methoden der Enzymatischen Analyse. Verlag Chemie, Weinheim, pp 454–558
Bergmeyer HU, Grassl M (1983) Reagents for enzymatic analysis; enzyme-α-amylase. In: Bergmeyer HU (ed) Methods of enzymatic analysis — II. 3rd ed, Verlag Chemie, Weinheim, FRG, pp 151–152
De Mot R, Andries K, Verachtert H (1984a) Comparative study of starch degradation and amylase production by ascomycetous yeast species. Syst Appl Microbiol 5:106–118
De Mot R, van Oudendijck E, Verachtert H (1984b) Production of extracellular debranching activity by amylolytic yeasts. Biotechnol Lett 6:581–586
Enevoldsen BS, Reimann L, Hansen NL (1977) Biospecific affinity chromatography of pullulanase. FEBS Lett 79:121–124
Hyun HH, Zeikus JG (1985a) General biochemical characterization of thermostable pullulanase and glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol 49:1168–1173
Hyun HH, Zeikus JG (1985b) Simultaneous and enhanced production of thermostable amylase and ethanol from starch by cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol 49:1174–1181
Jensen BF, Norman BE (1984) Bacillus acidopullulyticus pullulanase-application and regulatory aspects for use in the food industry. Proc Biochem 19:129–134
Konishi Y, Amemura A, Tanabe S, Harada T (1979) Immunological study of pullulanase from Klebsiella strains and the occurrence of this enzyme in the Enterobacteriaceae. Int J Syst Bacteriol 29:13–18
Madi E, Antranikian G, Ohmiya K, Gottschalk G (1987) Thermostable amylolytic enzymes from a new Clostridium isolate. Appl Environ Microbiol, in press
Nakamura N, Watanabe K, Horikoshi K (1975) Purification and some properties of alkaline pullulanases from a strain of Bacillus No. 202-1, an, alkalophilic microorganism. Biochem Biophys Acta 379:188–193
Plant AR, Morgan HW, Daniel RM (1986) A highly stable pullulanase from Thermus aquaticus YT-1. Enzyme Microb Technol 8:668–672
Suzuki Y, Chishiro M (1983) Production of extracellular thermostable pullulanase by an amylolytic obligately thermophilic soil bacterium, Bacillus stearothermophilus. K.P. 1064. Eur J Appl Biotechnol 17:24–29
Takasaki Y (1976a) Productions and utilizations of β-amylase and pullulanase from Bacillus cereus var. mycoides. Agr Biol Chem 40:1515–1522
Takasaki Y (1976b) Purification and enzymatic properties of β-amylase and pullulanase from Bacillus cereus var. mycoides. Agr Biol Chem 40:1523–1530
Takizawa N, Murooka Y (1985) Cloning of the pullulanase gene in Escherichia coli and Klebsiella aerogenes. Appl Environ Microbiol 49:294–298
Ueda S, Nanri N (1967) Production of isoamylase by Escherichia intermedia. Appl Environ Microbiol 15:492–496
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koch, R., Zablowski, P. & Antranikian, G. Highly active and thermostable amylases and pullulanases from various anaerobic thermophiles. Appl Microbiol Biotechnol 27, 192–198 (1987). https://doi.org/10.1007/BF00251944
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00251944