Summary
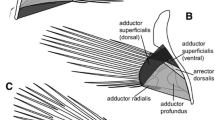
The metabolic and structural differentiation of locomotory muscles of Notothenia rossii has been investigated. In this species sustained locomotion is achieved by sculling with enlarged pectoral fins (labriform locomotion), whilst the segmental myotomal muscle is reserved for burst activity. Red, white and subepidermal fibres can be distinguished in the trunk by histochemical and ultrastructural criteria. The main pectoral muscle (m. adductor profundus) consists entirely of red fibres. These three main fibres types show differences in histochemical staining profiles, capillarization, myofibril shape and packing, and lipid and mitochondrial content. The fractional volume of mitochondria amounts to 38% for pectoral, 30% for red myotomal and 1.9% for white myotomal fibres. Enzyme activities of red pectoral muscle are consistent with a higher potential for aerobic glucose and fatty acid oxidation than for the red myotomal fibres. Mg2+ Ca2+ -myofibrillar ATPase activities are similar for red pectoral and myotomal muscles and approximately half of those white fibres. Specialisations of N. rossii muscles associated with labriform swimming and locomotion at Antarctic temperatures are discussed.
Similar content being viewed by others
References
Bainbridge R (1958) The speed of swimming of fish as related to the size and to the frequency and amplitude of tailbeat. J Exp Biol 35:109–133
Bainbridge R (1961) Problems of fish locomotion. Symp Zool Soc Lond 5:13–32
Bárány M (1967) ATPase activity of myosin correlated with speed of shortening. J Gen Physiol 50:197–218
Bass A, Brdiczka D, Eyer P, Hofer S, Pette D (1969) Metabolic differentiation of distinct muscle types at the level of enzymatic organisation. Eur J Biochem 10:198–206
Bilinski E (1974) Biochemical Aspects of Fish Swimming. In: Malins DC, Sargent JR (eds) Biochemical and Biophysical Perspectives in Marine Biology: Academic Press, New York Vol 1, pp 239–288
Bishop CM, Odense PH (1967) The ultrastructure of the white striated myotomal muscle of the cod, Gadus morhua. J Fish Res Bd Canada 24:2549–2553
Boddeke R, Slijper EJ, Stelt A Van der (1959) Histological characteristics of the body musculature of fishes in connection with their mode of life. Koninklijke Ned Akad von Wetenschappen Ser C 42:576–587
Bokdawala GD (1967) A histochemical study of fat in the red and white fibres of fish skeletal muscle. J Anim Morphol Physiol 154:231–241
Bone Q (1966) On the function of the two types of myotomal muscle fibre in elasmobranch fish. J Mar Biol Ass UK 46:321–349
Bone Q (1978) Locomotor muscle. In: Hoar WS, Randall DJ (eds) Fish Physiology. Academic Press, New York, Vol 7, pp 361–424
Bone Q, Kicenuik J, Jones DR (1978) On the role of different fibre types in fish myotomes at intermediate swimming speeds. Fish Bull 76:691–699
Bostrom SL, Johansson RG (1972) Enzyme activity patterns in white and red muscle of the eel (Anguilla anguilla) at different developmental stages. Comp Biochem Physiol 42B:533–542
Breder CM (1926) The locomotion of fishes. Zoologica (NY) 4:159–297
Chapell JB, Perry SV (1954) Creatine phosphokinase: assay and application for the microdetermination of adenine nucleotides. Biochem J 57:421–427
Crabtree B, Newsholme EA (1972) The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and glycerol-3-phosphate dehydrogenase in muscles from vertebrates and invertebrates. Biochem J, 126:49–58
DeVries AL, Eastman JR (1978) Lipid sacs as a buoyancy adaptation in Antarctic fish. Nature (Lond) 271:352–353
DeWitt HH (1971) Coastal and deep-water benthic fishes of the Antarctic. Antarctic Map Folio Series. American Geographical Society, New York, Folio 15:pp 1–10
Eggleton P, Elsden SR, Gough N (1943) The estimation of creatine and diacetyl. Biochem J 37:526–534
Ells HA (1959) A colorimetric method for the assay of soluble succinic dehydrogenase and pyridine nucleotide-linked dehydrogenases. Arch Biochem Biophys 85:561–565
Emery AR (1969) Comparative ecology of damselfishes (Pisces: Pomacentridae) at Alligator Reef, Florida Keys. Diss Abstr B 29:2962
Emery AR (1973) Comparative ecology and functional osteology of fourteen species of damsel fish (Pisces: Pomacentridae) at Alligator Reef, Florida Keys. Bull Mar Sci 23:649–770
Flitney FW (1971) The volume of the T-system and its association with the sarcoplasmic reticulum in slow muscles fibres of the frog. J Physiol (Lond) 217:243–257
Flitney FW, Johnston IA (1979) Mechanical properties of isolated fish red and white muscle fibres. J Physiol (Lond) 295:49–50P
Franzini-Armstrong C, Porter KR (1964) Sarcolemmal invaginations constituting the T-system in fish muscle fibres. J Cell Biol 22:675–696
George JC (1962) A histophysiological study of the red and white muscles of the mackerel. Am Midi Nat 68:486–494
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Gray J (1968) Animal Locomotion. Weidenfeld and Nicolson, London
Guppy M, Hulbert WC, Hochachka PW (1979) Metabolic sources of heat and power in tuna muscles II. Enzyme and metabolite profiles. J Exp Biol 82:303–320
Hamoir G (1978) Différenciation protéinique des muscles striés blancs, jaunes et cardiaque d'un Poisson antarctique exempt de'hémoglobine, Champsocephalus gunnari. CR Acad Sci Paris Servi Dis 286:145–148
Hazel JR (1972) The effect of temperature acclimation upon succinic dehydrogenase activity from the epaxial muscle of common goldfish (Carassius carassius, L.) -I. Properties of the enzyme and the effect of lipid extraction. Comp Biochem Physiol 43B:837–861
Hollander H, Vaaland JL (1968) A reliable staining method for semi-thin sections in experimental neuroanatomy. Brain Res 10:120–126
Hudson RCL (1973) On the function of the white muscles in teleosts at intermediate swimming speeds. J Exp Biol 58:509–522
Hulbert WC, Moon TW (1978) A histochemical, light and electron microscopic examination of eel Anguilla rostrata red and white muscle. J Fish Biol 13:527–533
Humphrey CD, Pitman FE (1974) A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol 49:9–14
Itzhaki RF, Gill DM (1964) A microbiuret method for estimating proteins. Analyt Biochem 9:401–410
Johnston IA (1977) A comparative study of glycolysis in red and white muscles of the trout (Salmo gairdnerii) and mirror carp (Cyrpinus carpio). J Fish Biol 11:575–588
Johnston IA (1980) Specialisations of fish muscle. In: Development and specialization of fish muscle (Ed D.F. Goldspink) Soc Exp Biol Seminar Series Symposium Cambridge University Press. (in press)
Johnston IA, Moon TW (1980) Endurance exercise training in the fast and slow muscles of a teleost fish (Pollachius virens). J Comp Physiol 135:147–156
Johnston IA, Frearson N, Goldspink G (1972) Myofibrillar ATPase activities of red and white myotomal muscles of marine fish. Experientia 28:713–714
Johnston IA, Davison W, Goldspink G (1977) Energy metabolism of carp swimming muscles. J Comp Physiol 114:203–216
Kalckar HM (1947) Differential spectrophotometry of purine compounds by means of specific enzymes, III. Studies of the enzymes of purine metabolism. J Biol Chem 167:461–475
Kilarski W (1967) The fine structure of striated muscles in teleosts. Z Zellforsch 79:562–580
Korneliussen H (1972) Identification of muscle fibre types in “semithin” sections stained with p-phenylene diamine. Histochemie 32:95–98
Korneliussen H, Dahl HA, Paulsen JE (1978) Histochemical definition of muscle fibre types in the trunk musculature of a teleost fish (Cod, Gadus morhua, L.). Histochemistry 55:1–16
Kryvi H (1977) Ultrastructure of the different fibre types in axial muscles of the sharks Etmopterus spinax and Galeus melastomus. Cell Tissue Res 184:297–312
Kryvi H, Totland GK (1978) Histochemistry and structure of the locomotory muscles of the cartilaginous fish Chimaera monstrosa J Fish Biol 12:257–265
Lännergren J (1979) An intermediate type of muscle fibre in Xenopus laevis. Nature (Lond) 279:254–256
Lin Y, Dobbs GH, Devries AL (1974) Oxygen consumption and lipid content in red and white muscles of antarctic fish. J Exp Zool 189:379–386
Lindsey CC (1978) Form, function and locomotory habits. In: Hoar WS, Randall DJ (eds) Fish Physiology. Academic Press, New York, Vol 7, pp 1–100
Matsuura F, Hashimoto K (1954) Chemical studies on the red muscle (“chai”) of goldfishes. II. Determinations of the contents of haemoglobin, myoglobin and cytochrome c in the muscles of fishes. Bull Jpn Soc Scient Fish 20:308–312
McCallister CP, Page E (1973) Effects of thyroxin on ultrastructure of rat myocardial cells. A stereological study. J Ultrastruct 42:136–155
Nachlas MM, Tsuo KC, Souza E, Cheng CS, Seligman AM (1957) Cytochemical demonstration of succinate dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazone. J Histochem Cytochem 5:420–424
Nag AC (1972) Ultrastructure and adenosine triphosphatase activity of red and white muscle fibres of the caudal region of a fish, Salmo gairdnerii. J Cell Biol 55:42–57
Newsholme EA, Zammitt VA, Crabtree B (1978) The role of glucose and glycogen as fuels for muscle. Biochem Soc Trans 6:512–520
Nishihara H (1967) Studies on the fine structure of red and white fin muscles of the fish (Carassius auratus). Arch Histol Jpn 28:425–447
Page S (1965) A comparison of the fine structures of frog slow and twitch muscle fibres. J Cell Biol 26:477–491
Patterson S, Goldspink G (1972) The fine structure of the red and white muscle fibres of the coalfish (Gadus virens). Z Zellforsch 133:463–473
Patterson S, Johnston IA, Goldspink G (1975) A histochemical study of the lateral muscles of five teleost species. J Fish Biol 7:159–166
Pearse AGE (1960) Histochemistry. Theoretical and Applied 2nd edition. Churchill, London
Perry SV, Grey TC (1956) A study of the effects of substrate concentration and certain relaxing factors on the magnesium-activated myofibrillar adenosine triphosphatase. Biochem J 64:184–192
Peter JB, Jeffress RN, Lamb DR (1968) Exercise: effects on hexokinase activity in red and white skeletal muscle. Science (Wash) 160:200–201
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Biophys Biochem Cytol 10:308–312
Robbillard GS, Dayton PK (1969) Notes on the biology of the Chaenichthyid fish Pagetopis macropterus. Antarctc J US 4:304–306
Rockstein M, Herron PW (1951) Colorimetric determination of inorganic phosphate in microgram quantities. Analyt Chem 23:1500–1501
Siegel S (1956) Non-parametric statistics for the behavioural sciences. New York
Smiley KL jr, Berry AJ, Suelter CH (1967) An improved purification, crystallisation and some properties of rabbit muscle 5′-adenylic acid deaminase. J Biol Chem 242:2502–2506
Solaro RJ, Pang DC, Briggs FN (1971) Purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta 245:259–262
Spamer C, Pette D (1979) Activities of malate dehydrogenase, 3-hydroxyacyl-CoA dehydrogenase and fructose-1, 6-diphosphatase with regard to metabolic subpopulations of fast and slow twitch fibres in rabbit muscles. Histochemistry 60:9–19
Storey KB, Bailey E (1978) Intracellular distribution of enzymes associated with lipogenesis and gluconeogenesis in fat body of the adult cockroach, Periplaneta. Insect Biochem 8:125–131
Totland GK (1976) Three muscle fibre types in the axial muscles of Axolotl (Ambystoma americanum, Shaw). A quantitative light and electron microscopic study. Cell Tissue Res 168:65–78
Twelves EL (1972) Blood volume of two Antarctic fishes. Br Antarct Surv Bull 31:85–92
Uehara Y, Campbell GR, Burnstock G (1976) Muscle and its Innervation. An atlas of fine structure. Edward Arnold, London
Walesby NJ, Johnston IA (1979) Activities of some enzymes of energy metabolism in the fast and slow muscles of an Antarctic teleost fish (Notothenia rossii). Biochem Soc Trans 7:659–661
Walesby NJ, Nicol CJM, Johnston IA (1980) Metabolic differentiation of muscle fibres from a haemoglobinless (Champsocephalus gunnari) and a red-blooded (Notothenia rossii) Antarctic fish. Br Antarct Surv Bull 53:(in press)
Watson ML (1958) Staining of tissue sections for electron microscopy with heavy salts. J Biophys Biochem Cytol 4:475–485
Webb PW (1973) Kinematics of pectoral fin propulsion in Cymatogaster aggregata. J Exp Biol 59:697–710
Webb PW (1975) Hydrodynamics and energetics of fish propulsion. Bull Fish Res Board Can 190:1–159
Winterbottom R (1974) A descriptive synonomy of the striated muscles of the Teleostei. Proc Acad Natl Sci Philadelphia 125:225–317
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walesby, N.J., Johnston, I.A. Fibre types in the locomotory muscles of an antarctic teleost, Notothenia rossii . Cell Tissue Res. 208, 143–164 (1980). https://doi.org/10.1007/BF00234180
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00234180