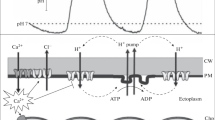
Abstract
The presence of calcium is essential for chloroplast movement induced by blue light in Lemna trisulca L. The regulatory role of calcium was confirmed by the inhibition of chloroplast movement by cytochalasin B and trifluoperazine. The calcium concentration in tissues was modified by ethylene glycol-bis(2-aminoethylether)-N,N,N′, N′-tetraacetic acid (EGTA), the calcium ionophore A23187 and La3+. Only a long period of incubation (12h) in EGTA or La3+ caused distrubances in chloroplast movement. This indicates that calcium influx is not essential for chloroplast movement. Those conditions that dramatically changed the internal calcium concentration, either applications of calcium ionophore A23187 and EGTA, or ionophore and La3+, markedly decreased the amplitude of response to blue-light pulses. This demonstrates that disturbances of chloroplast movement are observable only when internal stores of calcium are affected by Ca2+-antagonists. We suggest that the calcium involved in blue-light-induced chloroplast movement is derived from intracellular stores. The addition of Mg2+ to EGTA buffer counteracted its effect, indicating that Mg2+, as well as Ca2+, might possibly be involved in chloroplast movement.
Similar content being viewed by others
Abbreviations
- EGTA:
-
ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- Hepes:
-
4(2-hydroxyethyl-1-piperazine) ethanesulfonic acid
- A23187:
-
calcium ionophore
References
Äkerman, K.E.O., Proudlove, M.O., Moore, A.L. (1983) Evidence for a Ca2+ gradient across the plasma membrane of wheat protoplasts. Biochem. Biophys. Res. Commun. 113, 171–177
Amellal, M., Landry, Y. (1983) Lanthanides are transported by ionophore A 23187 and mimic calcium in the histamine secretion process. Br. J. Pharmac. 80, 365–370
Bonner, J., Galston, A.W. (1952) Principles of plant physiology. Freeman, San Francisco
Calbiochem, Biochemical/Immunochemical Catalog (1989), pp. 216–222
Campbell, A.K. (1983) Intracellular calcium. John Wiley & Sons, Inc., New York
Campbell, A.K., Siddle, K. (1976) The effect of intracellular calcium ions on adrenaline-stimulated adenosine 3′: 5′-cyclic monophosphate concentrations in pigeon erythrocytes: studies by using ionophore A 23187. Biochem. J. 158, 211–221
Campbell, A.K., Thomson, W.W. (1977) Effects of lanthanum and ethylenediaminetetraacetate on leaf movements of Mimosa. Plant Physiol. 60, 635–639
Chlobowski, A. (1986) Photometric method in chloroplast movement investigation. Photosynthetica 20, 486–493
Dreyer, E.M., Weisenseel, M.H. (1979) Phytochrome-mediated uptake of calcium in Mougeotia cells. Planta 146, 31–39
Gabryś, H., Walczak, T. (1980) Photometric study of chloroplast phototranslocations in leaves of land plants. Physiol. Plant. II, 281–290
Galland, P., Senger, H. (1988) The role of flavins as photoreceptors. J. Photochem. Photobiol. 1, 277–294
Gilroy, S., Hughes, W.A., Trewavas, A.J. (1989) A comparison between Quin-2 and aequorin as indicators of cytoplasmic calcium levels in higher plant cell protoplasts. Plant Physiol. 90, 482–491
Gilroy, S., Fricker, M.D., Read, N.D., Trewavas, A.J. (1991) Role of calcium in signal transduction of Commelina guard cells. Plant Cell. 3, 333–344
Grolig, F., Wagner, G. (1987) Vital staining permits isolation of the calcium vesicles from the green alga Mougeotia. Planta 171, 433–437
Grolig, F., Wagner, G. (1988) Light-dependent chloroplast rerientations in Mougeotia and Mesotaenium: biased by pigment-regulated plasmalemma anchorage sites to actin filaments? Bot. Acta 101, 2–6
Grolig, F., Wagner, G. (1989) Characterization of the isolated calcium-binding vesicles from the green alga Mougeotia scalaris, and their relevance to chloroplast movement. Planta 177, 169–177
Hart, J.W., Sabnis, D.D. (1976) Colchicine and plant microtubules: A critical evaluation. Curr. Adv. Plant. Sci. 8, 1095–1104
Haupt, W. (1959) Die Chloroplastendrehung bei Mougeotia. I. Über den quantitativen und qualitativen Lichtbedarf der Schwachlichtbewegung. Planta 53, 484–501
Haupt, W. (1983) Movement of chloroplasts under the control of light. Prog. Physiol. Res. 2, 227–281
Haupt, W., Scheuerlein, R. (1990) Chloroplast movement. Plant Cell Environ. 13, 595–614
Haupt, W., Wagner, G. (1984) Chloroplast movement. In: Membranes and sensory transduction, pp. 331–3375, Colombetti, G., Lenci, F., eds. Plenum, New York
Haupt, W., Weisenseel, M.H. (1976) Physiological evidence and some thoughts on localized responses, intracellular localization and action of phytochrome. In: Light and plant development, pp. 63–74, Smith, H., ed. Butterworths, Boston
Hepler, P., Palevitz, B. (1974) Microtubules and microfilaments. Annu. Rev. Plant Physiol. 25, 309–362
Herth, W. (1978) Ionophore A 23187 stops tip growth, but not cytoplasmic streaming, in pollen tubes of Lilium longiflorum. Protoplasma 96, 275–282
Inagaki, M., Tanaka, T., Sasaki, Y., Hidaka, H. (1985) Calcium dependent interactions of an ionophore A 23187 with calmodulin. Biochem. Biophys. Res. Commun. 130, 200–206
Jacobshagen, S., Altmüller, D., Grolig, F., Wagner, G. (1986) Calcium pools, calmodulin and light-regulated chloroplast movements in Mougeotia and Mesotaenium. In: Molecular and cellular aspects of calcium in plant development, pp. 201–209, Trewavas, A.J., ed. Plenum, New York
Lettvin, J.Y., Pickard, W.F., McCulloch, W.S., Pitts, W. (1964) A theory of passive ion flux through axon membranes. Nature 202, 1338–1339
Lew, R.R., Serlin, B.S., Schauf, C.L., Stockton, M.E. (1990) Calcium activation of Mougeotia potassium channels. Plant Physiol. 92, 831–836
Lynch, T.J. (1979) Human erythrocyte Ca2+-Mg2+-ATPase: mechanism of stimulation by Ca2+. Arch. Biochem. Biophys. 194, 165–179
Pfeiffer, D.R., Reed, P.W., Lardy, H.A. (1974) Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A 23187 and its metal ion complexes. Biochemistry 13, 4007–4014
Reid, R.J., Smith, F.A. (1992) Measurement of calcium fluxes in plants using 45Ca. Planta 186, 558–556
Russ, U., Grolig, F., Wagner, G. (1991) Changes of cytoplasmic free Ca2+ in the green alga Mougeotia scalaris as monitored with indo-1, and their effect on the velocity of chloroplast movements. Planta 184, 105–112
Schönbohm, E., Meyer-Wegener, J., Schönbohm, E. (1990) No evidence for influx as an essential link in the signal transduction chains of either light-oriented chloroplast movements or Pfr-mediated chloroplast anchorage in Mougeotia. J. Photochem. Photobiol. 5, 331–341
Serlin, B.S., Ferrel, S. (1991) The involvement of microtubules in chloroplast rotation in the alga Mougeotia. Plant Sci. 60, 1–8
Serlin, B.S., Roux, S.J. (1984) Modulation of chloroplast movement in the green alga Mougeotia by the Ca2+ ionophore A 23187 and by calmodulin antagonists. Proc. Natl. Acad. Sci. USA 81, 6368–6372
Thomson, W.W., Platt, K.A., Campbell, N. (1973) The use of lanthanum to delineate the apoplastic continuum in plants. Cytobios 8, 57–62
Tretyn, A., Kendrick, R.E., Wagner, G. (1991) The role(s) of calcium ions in phytochrome action. Photochem. Photobiol. 54, 1135–1155
Wagner, G., Bellini, E. (1976) Light-dependent fluxes and compartmentation of calcium in the green alga Mougeotia. Z. Pflanzenphysiol. 79, 283–291
Wagner, G., Klein, K. (1978) Differential effects of calcium on chloroplast movement in Mougeotia. Photochem. Photobiol. 27, 137–140
Wagner, G., Klein, K. (1981) Mechanism of chloroplast movement in Mougeotia. Protoplasma 109, 169–185
Wagner, G., Rossbacher, R. (1980) X-ray microanalysis and chlorotetracycline staining of calcium vesicles in the green alga Mougeotia. Planta 149, 298–305
Wagner, G., Grolig, F. (1985) Molecular mechanisms of photoinduced chloroplast movement. In: Sensory perception and transduction in aneural organisms, pp. 281–298, Colombetti, G., Lenci, F., Song, P.S., eds. Plenum Press, New York
Wagner, G., Grolig, F., Altmüller, D. (1987) Transduction chain of low irradiance response of chloroplast reorientation in Mougeotia in blue or red light. Photochem. Photobiophys. Suppl. 183–189
Wagner, G., Valentin, P., Dieter, P., Marmé, D. (1984) Identification of calmodulin in the green alga Mougeotia and its possible function in chloroplast reorientational movement. Planta 162, 62–67
Walczak, T., Gabryś, H. (1980) New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica 14, 65–72
Wayne, R., Hepleŕ, P.K. (1984) The role of calcium ions in phytochrome germination of Onoclea sensibilis L. Planta 160, 12–20
Wayne, R., Hepler, P.K. (1985) Red light stimulates an increase in intracellular calcium in the spores of Onoclea sensibilis. Plant Physiol. 77, 8–11
Weidinger, M., Ruppel, H.G. (1985) Ca2+-requirement for a bluelight-induced chloroplast translocation in Eremosphaera viridis. Protoplasma 124, 184–187
Weiss, G.B. (1974) Cellular pharmacology of lanthanum. Annu. Rev. Pharmacol. 14, 343–354
Zurzycki, J. (1962) The actin spectrum for the light dependent movements of chloroplasts in Lemna trisulca L. Acta Soc. Bot. Polon. 31, 489–538
Zurzycki, J. (1972) Primary reactions in the chloroplast rearrangements. Acta Protozool. 11, 189–200
Zurzycki, J. (1980) Blue-light-induced intracellular movements. In: The blue light syndrome, pp. 50–68, Senger, H., ed. Springer, Berlin Heidelberg New York
Zurzycki, J., Walczak, T., Gabryś, H., Kajfosz, J. (1983) Chloroplast translocations in Lemna trisulca L. induced by continuous irradiation and by light pulses. Kinetics analysis. Planta 157, 502–510
Author information
Authors and Affiliations
Additional information
We express our gratitude to Professor W. Korohoda for valuable critical comments on this paper and stimulating discussion. We also thank Mr. P. Malec for help in preparing the experiment with trifluoperazine and Mr. A. Waloszek for taking the photographs. We are indebted to Mr. Tim Kline (International House, Krakow, Poland) for improving the English style. This research was supported by grant No. 1042/P2/92/03 from the State Committe for Scientific Research.
Rights and permissions
About this article
Cite this article
Tlałka, M., Gabryś, H. Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L.. Planta 189, 491–498 (1993). https://doi.org/10.1007/BF00198211
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198211