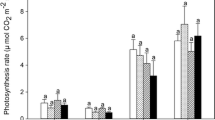
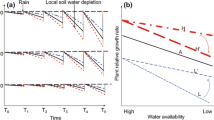
Abstract
Responses in stomatal conductance (g st ) and leaf xylem pressure potential (ψ leaf ) to elevated CO2 (2x ambient) were compared among 12 tallgrass prairie species that differed in growth form and growth rate. Open-top chambers (OTCs, 4.5 m diameter, 4.0 m in height) were used to expose plants to ambient and elevated CO2 concentrations from April through November in undisturbed tallgrass prairie in NE Kansas (USA). In June and August, ψ leaf was usually higher in all species at elevated CO2 and was lowest in adjacent field plots (without OTCs). During June, when water availability was high, elevated CO2 resulted in decreased g st in 10 of the 12 species measured. Greatest decreases in g st (ca. 50%) occurred in growth forms with the highest potential growth rates (C3 and C4 grasses, and C3 ruderals). In contrast, no significant decrease in g st was measured in the two C3 shrubs. During a dry period in September, reductions in g st at elevated CO2 were measured in only two species (a C3 ruderal and a C4 grass) whereas increased g st at elevated CO2 was measured in the shrubs and a C3 forb. These increases in g st were attributed to enhanced ψ leaf in the elevated CO2 plants resulting from increased soil water availability and/or greater root biomass. During a wet period in September, only reductions in g st were measured in response to elevated CO2. Thus, there was significant interspecific variability in stomatal responses to CO2 that may be related to growth form or growth rate and plant water relations. The effect of growth in the OTCs, relative to field plants, was usually positive for g st and was greatest (>30%) when water availability was low, but only 6–12% when ψ leaf was high.
The results of this study confirm the importance of considering interactions between indirect effects of high CO2 of plant water relations and direct effects of elevated CO2 on g st , particularly in ecosystems such as grasslands where water availability often limits productivity. A product of this interaction is that the potential exists for either positive or negative responses in g st to be measured at elevated levels of CO2.
Similar content being viewed by others
References
BazzazF.A. 1990. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 21: 167–196.
BunceJ.A. 1992a. Light, temperature and nutrients as factors in photosynthetic adjustment to an elevated concentration of carbon dioxide. Physiol. Plant. 86: 173–179.
BunceJ.A. 1992b. Stomatal conductance, photosynthesis and respiration of temperate deciduous tree seedlings grown outdoors at an elevated concentration of carbon dioxide. Plant. Cell and Environ. 15: 541–549.
CeulemansR. & MousseauM. 1994. Effects of elevated atmospheric CO2 on woody plants. New Phytol. 127: 425–446.
CureJ.D. & AcockB. 1986. Crop responses to carbon dioxide doubling: a literature survey. Agric. For. Meteor. 38: 127–145.
DahlmanR.C. 1993. CO2 and plants: revisited. Vegetatio 104/105: 339–355.
EamusD. & EamusD. & JarvisP.G. 1989. The direct effects of increase in the global atmospheric CO2 concentration on natural and commercial temperate trees and forests. Adv. Ecol. Res. 19: 1–55.
FahnestockJ.T. & KnappA.K. 1994. Plant responses to selective grazing by bison: interactions between light, herbivory and water stress. Vegetatio 115: 123–131.
FreemanC.C. & HulbertL.C. 1985. An annotated list of the vascular flora of Konza Prairie Research Natural Area, Kansas. Trans. Kan. Acad. Science 88: 84–115.
GundersonC.A. & WullschlegerS.D. 1994. Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photosyn. Res. 39: 369–388.
HamJ.M., OwensbyC.E., CoyneP.I. & BremerD.J. 1995. Fluxes of CO2 and water vapor from a prairie ecosystem exposed to ambient and elevated CO2. Argic. For. Meteor. 77: 73–93.
HuntR., HandD.W., HannahM.A. & NealA.M. 1991. Responses to CO2 enrichment in 27 herbaceous species. Funct. Ecol. 5: 410–421.
JacksonR.B., SalaO.E., FieldC.B. & MooneyH.A. 1994. CO2 alters water use, carbon gain, and yield for the dominant species in a natural grassland. Oecologia 98: 257–262.
KnappA.K. 1992. Leaf gas exchange in Quercus macrocarpa (Fagaceae): rapid stomatal responses to variability in sunlight in a tree growth form. Am. J. Bot. 79: 599–604.
KnappA.K. 1993. Gas exchange dynamics in C3 and C4 grasses: consequences of differences in stomatal conductance. Ecology 74: 113–123.
KnappA.K., HamerlynckE.P. & OwensbyC.E. 1993. Photosynthetic and water relations responses to elevated CO2 in the C4 grass Andropogon gerardii. Intl. J. Plant Sci. 154: 459–466.
KnappA.K., FahnestockJ.T. & OwensbyC.E. 1994. Elevated atmospheric CO2 alters stomatal responses to variable sunlight in a C4 grass. Plant, Cell and Environ. 17: 189–195.
KnappA.K. & SmithW.K. 1989. Influence of growth form and water relations on stomatal and photosynthetic responses to variable sunlight in subalpine plants. Ecology 70: 1069–1082.
LeadleyP.W. & DrakeB.G. 1993. Open top chambers for exposing plant canopies to elevated CO2 concentration and for measuring net gas exchange. Vegetatio 104/105: 3–15.
LuoY. & StrainB.R. 1992. Leaf water status in velvetleaf under long-term interactions of water stress, atmospheric humidity, and carbon dioxide. J. Plant Physiol. 139: 600–604.
MansfieldT.A., HetheringtonA.M. & AtkinsonC.J. 1990. Some current aspects of stomatal physiology. Annu. Rev. Plant Physiol. Plant Molec. Biol. 41: 55–75.
MasleJ. 1992. Will plant performance on soils prone to drought or with high mechanical impedance to root penetration be improved under elevated atmospheric CO2 concentration? Austl. J. Bot. 40: 491–500.
MorisonJ.I.L. 1993. Response of plants to CO2 under water limited conditions. Vegetatio 104/105: 193–209.
MorseS.R., WayneP., MiaoS.L. & BazzazF.A. 1993. Elevated CO2 and drought alter tissue water relations of birch (Betula populifolia Marsh.) seedlings. Oecologia 95: 599–602.
OwensbyC.E., CoyneP.I., HamJ.M., AuenL.M. & KnappA.K. 1993. Biomass production in a tallgrass prairie ecosystem exposed to ambient and elevated CO2. Ecol. Applic. 3: 644–653.
PaezA., HellmersH. & StrainB.R. 1983. CO2 enrichment, drought stress and growth of Alaska pea plants (Pisum sativum). Physiol. Plant. 58: 161–165.
ReginatoR.J. & VanBavelC.H.M. 1964. Soil water measurement with gamma attenuation. Soil Sci. Soc. Am. Proc. 28: 721–724.
RetuertoR. & WoodwardF.I. 1993. The influences of increased CO2 and water supply on growth, biomass allocation and water use efficiency of Sinapis alba L. grown under different wind speeds. Oecologia 94: 415–427.
RogersH.H., RunionG.B. & KrupaS.V. 1994. Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollut. 83: 155–189.
RogersH.H., SionitN., CureJ.D., SmithJ.M. & BinghamG.E. 1984. Influence of elevated carbon dioxide on water relations of soybeans. Plant Physiol. 74: 233–238.
SamuelsonL.J. & SeilerJ.R. 1993. Interactive role of elevated CO2, nutrient limitations, and water stress in the growth responses of red spruce seedlings. For. Sci. 39: 348–358.
SvejcarT.J. & BrowningJ.A. 1988. Growth and gas exchange of Andropogon gerardii as influenced by burning. J. Range Manage. 41: 239–244.
ThomasR.B. & StrainB.R. 1991. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol. 96: 627–634.
TschaplinskiT.J., NorbyR.J. & WullschlegerS.D. 1993. Responses of loblolly pine seedlings to elevated CO2 and fluctuating water supply. Tree Physiol. 13: 283–296.
TurnerC.T., KneislerJ.R. & KnappA.K. 1995. Comparative gas exchange and nitrogen responses of the dominant C4 grass, Andropogon gerardii, and five C3 forbs to fire and topographic position in tallgrass prairie during a wet year. Intl. J. Plant Sci. 156: 216–226.
TyreeM.T. & AlexanderJ.D. 1993. Plant water relations and the effects of elevated CO2: a review and suggestions for future research. Vegetatio 104/105: 47–62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knapp, A.K., Hamerlynck, E.P., Ham, J.M. et al. Responses in stomatal conductance to elevated CO2 in 12 grassland species that differ in growth form. Vegetatio 125, 31–41 (1996). https://doi.org/10.1007/BF00045202
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00045202