Abstract
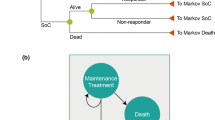
The National Institute for Health and Care Excellence (NICE) invited the manufacturer of ustekinumab (Janssen) to submit evidence for the clinical and cost effectiveness of ustekinumab for the treatment of active psoriatic arthritis (PsA) as part of the Institute’s single technology appraisal (STA) process. The Centre for Reviews and Dissemination and the Centre for Health Economics Technology Appraisal Group at the University of York were commissioned to act as the independent Evidence Review Group (ERG). This article provides a description of the ERG review of the manufacturer’s evidence submission, and summarises the NICE Appraisal Committee’s final guidance (TA340) issued in June 2015. The manufacturer presented evidence on ustekinumab for two patient populations: (1) a tumour necrosis factor-α (TNFα)-inhibitor-naïve population who had not previously received any TNFα inhibitors (biologics); and (2) a TNFα-inhibitor-exposed population who had previously received at least one TNFα inhibitor. The clinical evidence for ustekinumab was derived from two randomised controlled trials (PSUMMIT 1 and 2), in which a total of 927 patients who had not responded to previous disease-modifying antirheumatic drug therapies received ustekinumab 45 mg, ustekinumab 90 mg, or placebo. These data suggested that ustekinumab is more effective than placebo over 16–24 weeks in terms of both joint and skin response. In the absence of head-to-head comparisons between different biologics (ustekinumab, golimumab, etanercept, adalimumab and infliximab), the manufacturer conducted a network meta-analysis to estimate the relative efficacy of treatments for the TNFα-inhibitor-naïve population. Results of this analysis were marked as academic in confidence and are therefore not reported. For the TNFα-inhibitor-exposed population, the clinical analysis was limited to ustekinumab versus conventional management only, and was based on a subgroup of 180 patients from the PSUMMIT 2 trial. The ERG raised concerns relating to the lack of data on the long-term efficacy of ustekinumab, the limited data available for the exposed population, and the lack of consideration of the sequential use of treatments. Based on the manufacturer’s original model, the ERG found ustekinumab to be dominated by golimumab in the anti-TNF-inhibitor-naïve population, and had an incremental cost-effectiveness ratio (ICER) of £29,843/quality-adjusted life-years versus conventional management in the exposed population. The ERG’s analyses highlighted the fact that there is significant uncertainty around the model results. In addition, the ERG’s exploratory cost-effectiveness analysis, which incorporated the sequential use of TNFα inhibitors, suggested that ustekinumab would not be cost effective if it were used as second-line treatment. The initial NICE recommendations asserted that ustekinumab was not recommended for treating active PsA. However, the manufacturer submitted a post-consultation model that included a Patient Access Scheme (PAS), halving the unit cost of ustekinumab 90 mg to £2147 (the same as a 45 mg dose). The NICE final recommendations were that, dependent on the inclusion of the PAS, ustekinumab is recommended as an option, along or in combination with methotrexate, for treating active PsA in adults only when treatment with TNFα inhibitors is contraindicated but would otherwise be considered, or the person has previously had treatment with one or more TNFα inhibitors, which has failed.


Similar content being viewed by others
References
National Institute for Health and Care Excellence. Guide to the single technology appraisal (STA) process. London: NICE; 2006.
National Institute for Health and Care Excellence. TA340: ustekinumab for treating active psoriatic arthritis (rapid review of technology appraisal guidance 313). 2015. https://www.nice.org.uk/guidance/ta340. Accessed Aug 2015.
Craig D, O’Connor J, Rodgers M, et al. Ustekinumab for treating active and progressive psoriatic arthritis: a single technology appraisal, October 2013. London: NICE; 2013.
National Institute for Health and Care Excellence. TA220: golimumab for the treatment of psoriatic arthritis. London: NICE; 2011.
Ritchlin CT. From skin to bone: translational perspectives on psoriatic disease. J Rheumatol. 2008;35(7):1434–7.
National Institute for Health and Care Excellence. Biologic drugs for the treatment of inflammatory disease in rheumatology, dermatology and gastroenterology. Commissioning guide. London: NICE; 2012.
Leonard DG, O’Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalised for psoriasis. Mayo Clin Proc. 1978;53(8):511–8.
Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50(7):2264–72.
Zhu KJ, Zhu CY, Shi G, et al. Association of IL23R polymorphisms with psoriasis and psoriatic arthritis: a meta-analysis. Inflamm Res. 2012;61(10):1149–54.
National Institute for Health and Care Excellence. TA199: etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis. London: NICE; 2010.
National Institute for Health and Care Excellence. TA180: ustekinumab for the treatment of adults with moderate to severe psoriasis. London: NICE; 2009.
Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–9.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9.
Yang H, Craig D, Epstein D, Bojke L, Light K, Bruce IN, et al. Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal. Pharmacoeconomics. 2012;30:257–70.
National Health Service. Department of Health reference costs 2011–2012. 2012. http://www.dh.gov.uk/health/2012/11/2011-12-reference-costs/. Accessed Feb 2013.
Joint Formulary Committee. British National Formulary. 65th ed. Pharmaceutical Press; 2013.
Rodgers M, Epstein D, Bojke L, Yang H, Craig D, Fonseca T, et al. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. London: NICE; 2009.
Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. Outcomes after switching from one anti-tumour necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK cohort study. Arthritis Rheum. 2007;56:13–20.
Acknowledgments
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (project number 10/51/01) and has been published as part of a compendium of ERG articles in Health Technology Assessment (see the HTA programme website for further project information [http://www.hta.ac.uk]). This summary of the ERG report was compiled after the Appraisal Committee’s review. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NICE or the Department of Health.
The ERG would like to thank Ian Bruce for providing clinical advice throughout the project.
This summary has not been externally peer reviewed by PharmacoEconomics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Joanne O’Connor, Stephen Rice, Alison Smith, Mark Rodgers, Rocio Rodriguez Lopez, Dawn Craig and Nerys Woolacott have no competing interests.
Contributors
Joanne O’Connor, Alison Smith, Dawn Craig, Mark Rodgers, Nerys Woolacott and Rocio Rodriguez Lopez all formed part of the ERG that produced the ERG report that this paper describes. Stephen Rice contributed to later ERG submissions that are included in this paper. Joanne O’Connor, Alison Smith and Stephen Rice wrote the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript. Joanne O’Connor is the guarantor for the overall content.
Rights and permissions
About this article
Cite this article
O’Connor, J., Rice, S., Smith, A. et al. The Clinical and Cost Effectiveness of Ustekinumab for the Treatment of Psoriatic Arthritis: A Critique of the Evidence. PharmacoEconomics 34, 337–348 (2016). https://doi.org/10.1007/s40273-015-0350-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0350-3