Abstract
Background
Intravenous (IV) drug delivery is commonly used for its rapid administration and immediate drug effect. Most studies compare IV to subcutaneous (SC) delivery in terms of safety and efficacy, but little is known about what patients prefer.
Methods
A systematic review was conducted by searching seven electronic databases for articles published up to February 2014. Included studies were randomized controlled trials (RCTs) and/or crossover designs investigating patient preference for SC versus IV administration. The risk of bias in the RCTs was determined using the Cochrane Collaboration tool. Reviewers independently extracted data and assessed the risk of bias. Any discrepancies were resolved by consensus.
Results
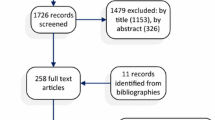
The search identified 115 publications, but few (6/115) met the inclusion criteria. Patient populations and drugs investigated were diverse. Four of six studies demonstrated a clear patient preference for SC administration. Main factors associated with SC preference were time saving and the ability to have treatment at home. Only three studies used study-specific instruments to measure preference.
Conclusions
Results suggest that patients prefer SC over IV delivery. Patient preference has clearly been neglected in clinical research, but it is important in medical decision making when choosing treatment methods as it has implications for adherence and quality of life. If the safety and efficacy of both administration routes are equivalent, then the most important factor should be patient preference as this will ensure optimal treatment adherence and ultimately improve patient experience or satisfaction. Future drug efficacy and safety studies should include contemporaneous, actual patient preference where possible, utilizing appropriate measures.

Similar content being viewed by others
References
Josephson D. Patient preparation and site selection for peripheral IV infusion therapy. Intravenous infusion therapy for nurses: principles and practice. 2nd ed. New York: Thomson Delmare Learning; 2004. p. 143.
Shapiro RS. Why I use subcutaneous immunoglobulin (SCIG). J Clin Immunol. 2013;33(Suppl 2):95–8.
Besarab A, Reyes CM, Hornberger J. Meta-analysis of subcutaneous versus intravenous epoetin in maintenance treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002;40:439–46.
Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109:1556–61.
Mazer M, Chen E. Is subcutaneous administration of rapid-acting insulin as effective as intravenous insulin for treating diabetic ketoacidosis? Ann Emerg Med. 2009;53:259–63.
Challiner YC, Jarrett D, Hayward MJ, al-Jubouri MA, Julious SA. A comparison of intravenous and subcutaneous hydration in elderly acute stroke patients. Postgrad Med J. 1994;70:195–7.
The Matisse Investigators. Subcutaneous fondaparinux versus unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–702.
Elsner F, Radbruch L, Loick G, Gaertner J, Sabatowski R. Intravenous versus subcutaneous morphine titration in patients with persisting exacerbation of cancer pain. J Palliat Med. 2005;8:743–50.
Gurpide A, Sadaba B, Martin-Algarra S, Azanza JR, Lopez-Picazo JM, Campanero MA, et al. Randomized crossover pharmacokinetic evaluation of subcutaneous versus intravenous granisetron in cancer patients treated with platinum-based chemotherapy. Oncologist. 2007;12:1151–5.
Kostic MA, Gutierrez FJ, Rieg TS, Moore TS, Gendron RT. A prospective, randomized trial of intravenous prochlorperazine versus subcutaneous sumatriptan in acute migraine therapy in the emergency department. Ann Emerg Med. 2010;56:1–6.
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40.
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–78.
Aron A, Wang J, Collier B, Ahmed N, Brateanu A. Subcutaneous versus intravenous insulin therapy for glucose control in non-diabetic trauma patients. A randomized controlled trial. J Clin Pharm Ther. 2013;38:24–30.
Keystone EC, Kremer JM, Russell A, Box J, Abud-Mendoza C, Elizondo MG, et al. Abatacept in subjects who switch from intravenous to subcutaneous therapy: results from the phase IIIb ATTUNE study. Ann Rheum Dis. 2012;71:857–61.
Nikas SN, Voulgari PV, Alamanos Y, Papadopoulos CG, Venetsanopoulou AI, Georgiadis AN, et al. Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study. Ann Rheum Dis. 2006;65:257–60.
Davies A, Merli F, Mihaljevic B, Siritanaratkul N, Solal-Céligny P, Barrett M, et al. Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): stage 1 analysis of a randomised phase 3 study. Lancet Oncol. 2014;15(3):3543–52.
Beerse, Belgium. Subcutaneous Velcade approved in the EU for the treatment of multiple myeloma. Published 28/09/2012. http://www.investor.jnj.com/releasedetail.cfm?ReleaseID=710106. Accessed 19 Mar 2014.
Allen PB, Lindsay H, Tham TCK. How do patients with inflammatory bowel disease want their biological therapy administered? BMC Gastroenterol. 2010;10:1.
Barbee MS, Harvey RD, Lonial S, Kaufman JL, Wilson NM, McKibbin T, et al. Subcutaneous versus intravenous bortezomib: efficiency practice variable and patient preferences. Ann Pharmacother. 2013;47:1136–42.
Williams EL, Edwards CJ. Patient preferences in choosing anti-TNF therapies-R1. Rheumatology. 2006;45:1575–6.
Scarpato S, Antivalle M, Favalli EG, Nacci F, Frigelli S, Bartoli F, et al. Patient preferences in the choice on anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology. 2010;49:289–94.
Desai SH, Chouksey A, Poll J, Berger M. A pilot study of equal doses of 10 % IGIV given intravenously or subcutaneously. J Allergy Clin Immunol. 2009;124:854–6.
Soremekun OA, Shear ML, Patel S, Kim GJ, Biddinger PD, Parry BA, et al. Rapid vascular glucose uptake via enzyme-assisted subcutaneous infusion: enzyme-assisted subcutaneous infusion access study. Am J Emerg Med. 2009;27:1072–80.
Matza LS, Cong Z, Chung K, Stopeck A, Tonkin K, Brown J, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adherence. 2013;7:855–65.
Sibbald B, Roland M. Understanding controlled trials: why are randomised controlled trial important? BMJ. 1998;316:201.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. http://www.cochrane-handbook.org. Accessed 19 Mar 2014.
Robinson AM, McLean KA, Greaves M, Channer KS. Subcutaneous versus intravenous administration of heparin in the treatment of deep vein thrombosis; which do patients prefer? A randomized cross-over study. Postgrad Med J. 1993;69:115–6.
Assche G, Vermeire S, Ballet V, Gabriels F, Noman M, D’Haens G, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut. 2012;61:229–34.
Harbo T, Andersen H, Hess A, Hansen K, Sindrup SH, Jakobsen J. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. Eur J Neurol. 2009;16:631–8.
Pivot X, Gligorov J, Müller V, Barrett-Lee P, Verma S, Knoop A, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–70.
Urquhart ML, Klapp K, White PF. Patient-controlled analgesia: a comparison of intravenous versus subcutaneous hydromorphone. Anesthesiology. 1988;69:428–32.
Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100.
Bjoerner JB, Damsgaard MT, Watt T, Bech P, Rasmusen NK, Kristensen TS, et al. Danish manual for SF-36. Copenhagen: Lif Lægemiddelindustriforeningen; 1997.
Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–10.
Kawata AK, Kieinman L, Harding G, Ramachandran S. Evaluation of patient preference and willingness to pay for attributes of maintenance medication for chronic obstructive pulmonary disease (COPD). Patient. 2014;. doi:10.1007/s40271-014-0064-1.
Johnson P, Bancroft T, Barron R, Legg J, Li X, Watson H, et al. Discrete choice experiment to estimate breast cancer patients’ preferences and willingness to pay for prophylactic granulocyte colony-stimulating factors. Value Health. 2014;17(4):380–9.
Huynh TK, Østergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biological agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence. 2014;20(8):93–9.
Abolhassani H, Sadaghiani MS, Aghamohammadi A, Ochs HD, Rezaei N. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta-analysis. J Clin Immunol. 2012;32:1180–92.
Conflict of interest
Miss Kelly Stoner, Dr Helena Harder, Professor Lesley Fallowfield, and Dr Valerie Jenkins have no conflicts of interest to declare. SHORE-C received funding from Hoffmann-La Roche to conduct the PREFER study. Kelly Stoner and Helena Harder conducted the literature search and article selection. All authors contributed equally to data interpretation and writing of the systematic review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoner, K.L., Harder, H., Fallowfield, L.J. et al. Intravenous versus Subcutaneous Drug Administration. Which Do Patients Prefer? A Systematic Review. Patient 8, 145–153 (2015). https://doi.org/10.1007/s40271-014-0075-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-014-0075-y



