Abstract
Background and Objective
The skeletal muscle relaxant tizanidine is approved by the US FDA and the European Medicines Agency for treating spasticity and is supplied as tablets for oral administration. However, tizanidine has a poor bioavailability, due to extensive first-pass metabolism. Therefore, the nasal route of administration, which bypasses portal circulation, may increase the bioavailability of tizanidine and, possibly, reduce the time to peak plasma concentration, thereby shorting the latency of therapeutic effect. The objective of this study was to evaluate the pharmacokinetic profile of tizanidine nasal spray and compare it to the profile of tizanidine oral tablets.
Methods
This open-label, phase I study comprised two protocols: protocol 1, tizanidine HCl solution (32.73 mg/mL) intranasally at single doses of 2 and 4 mg versus 4 mg tizanidine oral tablets (randomized, three periods crossover, 12 healthy subjects); and protocol 2, tizanidine HCl solution (16.36 mg/mL) intranasally at a single dose of 1 mg vs. 4 mg tizanidine oral tablets (randomized, two periods crossover, 12 healthy subjects, one dropout). Tizanidine plasma concentrations were determined by liquid chromatography/mass spectrometry.
Results
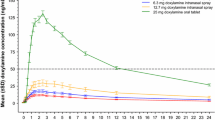
There was a linear relationship between different dosages of intranasal formulation and the area under the concentration–time curve and maximum plasma concentration (C max). The relative bioavailability of the different dosages of intranasal formulation were 1.29, 1.93, and 4.23 for 1, 2, and 4 mg intranasal administration, respectively. Comparison of C max values gave the following ratios: 0.91, 1.39, and 2.73, for 1, 2, and 4 mg intranasal administration, respectively. The mean time to C max (t max) was 0.99, 0.43, and 0.63 h for 1, 2, and 4 mg intranasal administration, respectively, whereas it was 1.13 and 1.30 h for the two series of 4 mg tizanidine oral tablets.
Conclusions
The bioavailability of the tizanidine intranasal formulation was higher than that of tizanidine oral tablets. The t max was also shorter with the intranasal formulation. No serious adverse events occurred throughout the study, such that the two formulations resulted equally well-tolerated. The intranasal formulation of tizanidine results are therefore worthy of subsequent clinical testing in phase II.


Similar content being viewed by others
References
John P, Khan IU, Akkurt M, et al. 5-Chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-2,1,3-benzothia-diazol-4-amine (tizanidine). Acta Crystallogr Sect E Struct Rep Online. 2011;67:o838–9.
Wagstaff AJ, Bryson HM. Tizanidine: a review of its pharmacology, clinical efficacy and tolerability in the management of spasticity associated with cerebral and spinal disorders. Drugs. 1997;53:435–52.
Lake AE 3rd, Saper JR. Chronic headache: new advances in treatment strategies. Neurology. 2002;59:S8–13.
Muramatsu I, Kigoshi S. Tizanidine may discriminate between imidazoline-receptors and alpha 2-adrenoceptors. Jpn J Pharmacol. 1992;59:457–9.
Honda M, Sekiguchi Y, Sato N, et al. Involvement of imidazoline receptors in the centrally acting muscle-relaxant effects of tizanidine. Eur J Pharmacol. 2002;445:187–93.
Bousquet P, Greney H, Bruban V, et al. I(1) imidazoline receptors involved in cardiovascular regulation: where are we and where are we going? Ann N Y Acad Sci. 2003;1009:228–33.
Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2011;2:9.
Delwaide PJ, Pennisi G. Tizanidine and electrophysiologic analysis of spinal control mechanisms in humans with spasticity. Neurology. 1994;44:S21–7.
Danzebrink RM, Gebhart GF. Antinociceptive effects of intrathecal adrenoceptor agonists in a rat model of visceral nociception. J Pharmacol Exp Ther. 1990;253:698–705.
Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:1–111.
Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage. 2004;28:140–75.
Dones I, Nazzi V, Broggi G. The guidelines for the diagnosis and treatment of spasticity. J Neurosurg Sci. 2006;50:101–5.
Hoogstraten MC, van der Ploeg RJ, vd Burg W, et al. Tizanidine versus baclofen in the treatment of spasticity in multiple sclerosis patients. Acta Neurol Scand. 1988;77:224–30.
Delwaide PJ. Electrophysiological analysis of the mode of action of muscle relaxants in spasticity. Ann Neurol. 1985;17:90–5.
Lataste X, Emre M, Davis C, et al. Comparative profile of tizanidine in the management of spasticity. Neurology. 1994;44:S53–9.
Knutsson E, Martensson A, Gransberg L. Antiparetic and antispastic effects induced by tizanidine in patients with spastic paresis. J Neurol Sci. 1982;53:187–204.
Mathias CJ, Luckitt J, Desai P, Baker H, el Masri W, Frankel HL. Pharmacodynamics and pharmacokinetics of the oral antispastic agent tizanidine in patients with spinal cord injury. J Rehabil Res Dev. 1989;26:9–16.
Berry H, Hutchinson DR. A multicentre placebo-controlled study in general practice to evaluate the efficacy and safety of tizanidine in acute low-back pain. J Int Med Res. 1988;16:75–82.
Tse FL, Jaffe JM, Bhuta S. Pharmacokinetics of orally administered tizanidine in healthy volunteers. Fundam Clin Pharmacol. 1987;1:479–88.
Heazlewood V, Symoniw P, Maruff P, et al. Tizanidine—initial pharmacokinetic studies in patients with spasticity. Eur J Clin Pharmacol. 1983;25:65–7.
Costantino HR, Illum L, Brandt G, et al. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337:1–24.
ICH. Guidance for industry: E6 good clinical practice: consolidated guidance. April 1996. http://www.fda.gov/downloads/Drugs/Guidances/ucm073122.pdf. Accessed 19 Sep 2013.
Committee for Proprietary Medicinal Products (CPMP). Note for guidance on the investigation of bioavailability and bioequivalence. CMP/EWP/QWP/1401/98. London: EMEA; 2000.
OECD Principles on Good Laboratory Practice. http://www.oecd.org/chemicalsafety/testing/oecdseriesonprinciplesofgoodlaboratorypracticeglpandcompliancemonitoring.htm. Accessed 19 Sep 2013.
Nirogi RV, Kandikere VN, Shukla M, et al. Quantification of tizanidine in human plasma by liquid chromatography coupled to tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2286–92.
Granfors MT, Backman JT, Laitila J, et al. Tizanidine is mainly metabolized by cytochrome p450 1A2 in vitro. Br J Clin Pharmacol. 2004;57:349–53.
Browning SL, Tarekegn A, Bekele E, et al. CYP1A2 is more variable than previously thought: a genomic biography of the gene behind the human drug-metabolizing enzyme. Pharmacogenet Genomics. 2010;20:647–64.
Momo K, Homma M, Osaka Y, et al. Effects of mexiletine, a CYP1A2 inhibitor, on tizanidine pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2010;50:331–7.
Neuvonen PJ. Towards safer and more predictable drug treatment—reflections from studies of the first BCPT prize awardee. Basic Clin Pharmacol Toxicol. 2012;110:207–18.
Granfors MT, Backman JT, Laitila J, et al. Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin Pharmacol Ther. 2005;78:400–11.
Granfors MT, Backman JT, Neuvonen M, et al. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76:598–606.
Granfors MT, Backman JT, Neuvonen M, et al. Fluvoxamine drastically increases concentrations and effects of tizanidine: a potentially hazardous interaction. Clin Pharmacol Ther. 2004;75:331–41.
Backman JT, Granfors MT, Neuvonen PJ. Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol. 2006;62:451–61.
Gelber DA, Good DC, Dromerick A, et al. Open-label dose-titration safety and efficacy study of tizanidine hydrochloride in the treatment of spasticity associated with chronic stroke. Stroke. 2001;32:1841–6.
Nance PW, Sheremata WA, Lynch SG, et al. Relationship of the antispasticity effect of tizanidine to plasma concentration in patients with multiple sclerosis. Arch Neurol. 1997;54:731–6.
Henney HR 3rd, Runyan JD. A clinically relevant review of tizanidine hydrochloride dose relationships to pharmacokinetics, drug safety and effectiveness in healthy subjects and patients. Int J Clin Pract. 2008;62:314–24.
Emre M, Leslie GC, Muir C, et al. Correlations between dose, plasma concentrations, and antispastic action of tizanidine (Sirdalud). J Neurol Neurosurg Psychiatry. 1994;57:1355–9.
Acknowledgments
This study was supported by MDM SpA. The authors do not have any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitale, D.C., Piazza, C., Sinagra, T. et al. Pharmacokinetic Characterization of Tizanidine Nasal Spray, a Novel Intranasal Delivery Method for the Treatment of Skeletal Muscle Spasm. Clin Drug Investig 33, 885–891 (2013). https://doi.org/10.1007/s40261-013-0137-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0137-2