Abstract
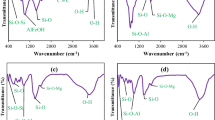
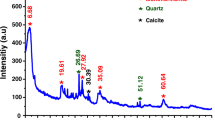
This paper assesses the use of certified Iraqi montmorillonite clay as a potential sorbent for the removal of oxytetracycline (OTC) from aqueous solutions. The clay is characterized by a cation exchange capacity of 0.756 meq g−1 and a zero point charge at pH 8.7. Aqueous solutions of OTC were equilibrated with montmorillonite under various experimental conditions, such as OTC concentration, pH and clay content, for 24 h at fixed ionic strength. Two forms of montmorillonite were evaluated: regular and iron-modified form. The effect of pH was minor on OTC adsorption. Kinetic study revealed that the sorption follows a pseudo-second-order model. Sorption isotherm showed a good fit with the Freundlich model. OTC sorption onto Fe-saturated montmorillonite was analyzed statistically using a response surface design to study the effects of experimental conditions. The introduction of iron improved the adsorption characteristics of the clay due to the ability of ferric ions to make stable complexes with OTC. The most favorable operating conditions for the treatment were deemed as follows: clay content, 6.85 g L−1, oxytetracycline concentration, 1.0 mmol L−1 and pH, 5.5 for the iron-modified form.








Similar content being viewed by others
References
Al-Asheh S, Duvnjak Z (1997) Sorption of cadmium and other heavy metals by pine bark. J Hazard Mater 56(1–2):35–51
Alwarthan AA, Al-Tamrah SA, Sultan SM (1991) Spectrophotometric determination of oxytetracycline by flow injection. Analyst 116(2):183–186
Arikan OA, Sikora LJ, Mulbry W, Khan SU, Rice C, Foster GD (2006) The fate and effect of oxytetracycline during the anaerobic digestion of manure from therapeutically treated calves. Process Biochem 41(7):1637–1643
Avisar D, Primor O, Gozlan I, Mamane H (2009) Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water Air Soil Pollut 209(1–4):439–450
Bansal OP (2012) Thermodynamics of equilibrium adsorption of antibiotics by clay minerals and humic acid–clay complexes. Nat Acade Sci Lett 35(2):109–114
Barbooti MM, Al-Bassam KS, Hussain BQ (2012) Evaluation of sorption characteristics of Iraqi montmorillonite. Iraqi J Sci 53(2):351–363
Campbell NRC, Hasinoff BB (1991) Iron supplements: a common cause of drug interactions. Br J Clin Pharmac 31(3):251–255
Chandran CB, Subramanian TV, Felse PA (2002) Parametric optimization of biotransportation of Cd by mixed function oxidase produced under controlled conditions in saccharomyces cerevisiae. Indian Chem Eng 44:223–229
Choi KJ, Kim SG, Kim SH (2008) Removal of antibiotics by coagulation and granular activated carbon filtration. J Hazard Mater 151(1):38–43
Doi AM, Stoskopf MK (2000) The kinetics of oxytetracycline degradation in deionized water under varying temperature, pH, light, substrate, and organic matter. J Aquat Animal Health 12(3):246–253
Duff A (2005) Presence of tetracycline antibiotics in surface water—a study of the presence/absence of tetracycline in the Raccoon River watershed, Des Moines Water Works Laboratory
Figueroa AL, Mackay A (2004) Modelling tetracycline antibiotic sorption to clays. Environ Sci Technol 38(2):476–483
Fritz JW, Zuo Y (2007) Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography. Food Chem 105(3):1297–1301
Gerstl Z, Banin A (1980) Fe2+-Fe3+ transformations in clay and resin ion-exchange systems. Clays Clay Miner 28(5):335–345
Gulkowska A, Leung HW, So MK, Taniyasu S, Yamashitab N, Yeung LWY, Richardson BJ, Lei AP, Giesye JP, Lama PKS (2008) Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res 42:395–403
Hayes PL, Geiger FM (2008) Oxytetracycline at Environmental Interfaces Studied by Second Harmonic generation, WMRC Symposium on PPCPs in the Illinois environment
Henry CM (2000) Antibiotic resistance. Chem Eng News 78(10):41–58
Ho YS (2005) Effect of pH on lead removal using tree fern as the sorbent. Bioresour Technol 96(11):1292–1296
Kim S, Eichhorn P, Jensen JN, Scott Weber A, Aga DS (2005) Removal of antibiotics in wastewater: effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ Sci Technol 39(15):5816–5823
Kulshrestha P, Giese RF Jr, Aga DS (2004) Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil. Environ Sci Technol 38(15):4097–4105
Lawson J (2009) Design and analysis of experiments with SAS. CRC Press, Boca Raton, FL
Lee W, Zhi-Hong L, Vakulenko S, Mobashery S (2000) A light-activated antibiotic. J Med Chem 43(1):128–132
Levy SB (1997) Antibiotic resistance: an ecological imbalance. In: Chadwick DJ, Goode F (eds) Antibiotic resistance: origins, evolution, selection and spread, vol 207. Ciba Foundation Symposium, Wiley, Chichester, pp 1–14
MacKay AA, Canterbury B (2005) Oxytetracycline sorption to organic matter by metal-bridging. J Environ Quality 34(6):1964–1971
Parolo ME, Savini MC, Vallés JM, Baschini MT, Avena MG (2008) Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl Clay Sci 40(1–4):179–186
Peterson JW, O’Meara TA, Seymour MD, Wang W, Gu B (2009) Sorption mechanisms of cephapirin, a veterinary antibiotic, onto quartz and feldspar minerals as detected by Raman spectroscopy. Environ Pollut 157(6):1849–1856
Polubesova T, Zadaka D, Groisman L, Nir S (2006) Water remediation by micelle–clay system: case study for tetracycline and sulfonamide antibiotics. Water Res 40:2369–2374
Pusino A, Pinna MV, Gessa C (2004) Azimsulfuron sorption-desorption on soil. J Agric Food Chem 52(11):3462–3466
Vu BK, Shin EW, Snisarenko O, Jeong WS, Lee HS (2010) Removal of the antibiotic tetracycline by Fe-impregnated SBA-15. Korean J Chem Eng 27(1):116–120
Watkinson AJ, Murby EJ, Costanzo SD (2007) Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res 41(18):4164–4176
Zhao Y, Geng J, Wang X, Gu X, Gao S (2011) Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicol 20:1141–1147
Acknowledgments
MMB acknowledges the Institute of International Education for supporting his scholarship and to the University of Technology, Iraq, for authorizing the scientific vacation to conduct this work in United States. Montmorillonite was supplied by Dr. Khaldoon Al-Bassam of the Iraqi Geological Survey; his assistance is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbooti, M.M., Su, H., Punamiya, P. et al. Oxytetracycline sorption onto Iraqi montmorillonite. Int. J. Environ. Sci. Technol. 11, 69–76 (2014). https://doi.org/10.1007/s13762-013-0361-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0361-6