Abstract
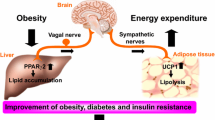
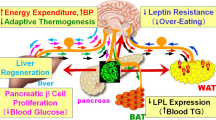
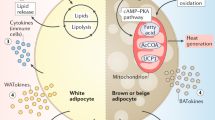
Obesity-associated diabetes, a major risk factor for numerous disorders including chronic renal, cardiovascular, and cerebrovascular diseases, is a rapidly increasing public health challenge worldwide. Despite several remarkable advances in obesity research over the past decade, the mechanisms underlying obesity are still not fully understood. In recent years it has been revealed that several metabolic interactions between organs and tissues, which are mediated by neuronal networks governed by the brain, function as a negative-feedback mechanism to maintain body weight homeostasis, even when energy intake is excessive. On the other hand, we recently discovered a new inter-organ neural network, from the liver, possibly representing a positive-feedback mechanism. In states of energy intake beyond physiological requirements, glucose metabolism changes in the liver with increased hepatic glucokinase expression and the induction of neuronal signal transmissions via the afferent vagal nerve. These signals, received by the medulla, result in inactivation of sympathetic innervation of brown adipose tissue (BAT). This leads to suppression of thermogenesis in BAT and thereby favors obesity development. This review focuses on the neuronal network mediating the inter-organ communication necessary for glucose and energy metabolism.



Similar content being viewed by others
References
Yamada T, Oka Y, Katagiri H. Inter-organ metabolic communication involved in energy homeostasis: potential therapeutic targets for obesity and metabolic syndrome. Pharmacol Ther. 2008;117:188–98.
Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53.
Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39.
Yamada T, et al. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 2006;3:223–9.
Uno K, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–9.
Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–14.
Shimazu T. Central nervous system regulation of liver and adipose tissue metabolism. Diabetologia. 1981;20(Suppl):343–56.
Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–411.
Imai J, et al. Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity (Silver Spring). 2006;14:1132–41.
Kreier F, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat–functional implications. J Clin Invest. 2002;110:1243–50.
Giordano A, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–55.
Kreier F, et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–7.
Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;253:R942–4.
Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst. 1998;73:19–25.
Tanida M, et al. Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci Lett. 2000;293:107–10.
Okamatsu-Ogura Y, et al. Possible involvement of uncoupling protein 1 in appetite control by leptin. Exp Biol Med (Maywood). 2011;236:1274–81.
Commins SP, et al. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300.
Plum L, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–45.
Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Opin Clin Nutr Metab Care. 2004;7:471–8.
Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–91.
Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med (Maywood). 2001;226:963–77.
Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522.
Smith GP, et al. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–7.
Koda S, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75.
Abbott CR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31.
Date Y, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8.
Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–63.
Gribble FM, et al. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54.
Abizaid A, Horvath TL. Ghrelin and the central regulation of feeding and energy balance. Indian J Endocrinol Metab. 2012;16:S617–26.
Burcelin R, Dolci W, Thorens B. Glucose sensing by the hepatoportal sensor is GLUT2-dependent: in vivo analysis in GLUT2-null mice. Diabetes. 2000;49:1643–8.
Burcelin R, et al. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–8.
Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46:1521–5.
Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature. 1963;197:79–80.
Russek M. Demonstration of the influence of an hepatic glucosensitive mechanism on food-intake. Physiol Behav. 1970;5:1207–9.
Gardemann A, Strulik H, Jungermann K. A portal-arterial glucose concentration gradient as a signal for an insulin-dependent net glucose uptake in perfused rat liver. FEBS Lett. 1986;202:255–9.
Cardin S, et al. Portal glucose infusion increases hepatic glycogen deposition in conscious unrestrained rats. J Appl Physiol. 1999;87:1470–5.
Burcelin R, Dolci W, Thorens B. Portal glucose infusion in the mouse induces hypoglycemia: evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes. 2000;49:1635–42.
Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514.
Feige JN, et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45:120–59.
Matsusue K, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–47.
Rahimian R, et al. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem. 2001;224:29–37.
Chao L, et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest. 2000;106:1221–8.
Yamada T, Katagiri H. Avenues of communication between the brain and tissues/organs involved in energy homeostasis. Endocr J. 2007;54:497–505.
Bernal-Mizrachi C, et al. An afferent vagal nerve pathway links hepatic PPARalpha activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metab. 2007;5:91–102.
Ferre T, et al. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia. 2003;46:1662–8.
Tsukita S, et al. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 2012;16:825–32.
Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31.
Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
Wijers SL, Saris WH, van Marken Lichtenbelt WD. Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity. Obes Rev. 2009;10:218–26.
Bouchard C, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–82.
West DB, et al. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–32.
Prpic V, et al. Adaptive changes in adipocyte gene expression differ in AKR/J and SWR/J mice during diet-induced obesity. J Nutr. 2002;132:3325–32.
Surwit RS, et al. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci USA. 1998;95:4061–5.
Acknowledgments
Parts of this review were presented as the Lilly Award Lecture at the Japan Diabetes Society 2013, Kumamoto, Japan. The author thanks Professors Hideki Katagiri and Yoshitomo Oka for their instruction and invaluable support. The significant contributions of colleagues and collaborators, especially Drs Yasushi Ishigaki, Kenji Uno, and Sohei Tsukita, are also deeply appreciated. The studies cited in this review were supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology and a Core Research for Evolutional Science and Technology grant from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamada, T. Inter-organ communications mediate crosstalk between glucose and energy metabolism. Diabetol Int 4, 149–155 (2013). https://doi.org/10.1007/s13340-013-0133-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-013-0133-z