Abstract
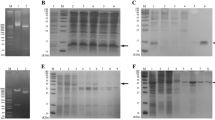
Anthrax lethal toxin (LeTx; a combination of protective antigen and lethal factor) is secreted by the vegetative cells of Bacillus anthracis and is cytotoxic for certain macrophage cell lines. First-time exposure of murine macrophage cells (RAW 264.7) to lethal toxin (LeTx) (0.1+0.1 mg/mL) caused extensive cell death with a survival rate of approximately 40%, but upon secondary exposure to LeTx and lipopolysaccharide (LPS) (1 μg/mL), after a few passages, these cells had a survival rate of approximately 100%. The present study assessed protein expression changes after LPS exposure to LeTx-intoxication-resistant RAW 264.7 cells. To analyze the protein expression profile of LPS-treated LeTx-intoxication-resistant RAW 264.7 cells, we employed matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDITOF MS), and later, Ingenuity Pathways Analysis (IPA) was applied to establish the molecular networks. Among the differentially expressed proteins were voltage dependent anion channel 1, jip3 protein, heat shock protein 4, tubulin beta, 26S protease regulatory subunit 4, and DNA polymerase delta subunit 4 (DNA polδ) were significant. The molecular network signatures and functional proteomics obtained in this study may facilitate the evaluation of potential pathways such as PI3K signaling, NF-κB signaling, and LPSinduced MAPK signaling for the recovery of LPSinduced LeTx-intoxication-resistant RAW 264.7 cells.
Similar content being viewed by others
References
Bonuccelli, G. et al. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis’ three sites of entry: implications for the pathogenesis of anthrax infection. Am. J. Physiol. Cell Physiol. 288, C1402–1410 (2005).
Rmali, K.A., Al-Rawi, M.A., Parr, C., Puntis, M.C. & Jiang, W.G. Upregulation of tumour endothelial marker-8 by interleukin-1beta and its impact in IL-1beta induced angiogenesis. Int. J. Mol. Med. 14, 75–80 (2004).
Boyden, E.D. & Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Muehlbauer, S.M. et al. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6, 758–766 (2007).
Ha, S.D., Ng, D., Pelech, S.L. & Kim, S.O. Critical role of the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3 signaling pathway in recovery from anthrax lethal toxin-induced cell cycle arrest and MEK cleavage in macrophages. J. Biol. Chem. 282, 36230–36239 (2007).
Tang, G. & Leppla, S.H. Proteasome activity is required for anthrax lethal toxin to kill macrophages. Infect. Immun. 67, 3055–3060 (1999).
Jope, R.S. & Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29, 95–102 (2004).
Baetz, D. et al. Nuclear factor-kappaB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes. Circulation 112, 3777–3785 (2005).
Das, N.D., Jung, K.H. & Chai, Y.G. The role of NFkappaB and H3K27me3 demethylase, Jmjd3, on the anthrax lethal toxin tolerance of RAW 264.7 cells. PLoS One 5, e9913 (2010).
Jung, K.H. et al. Effects of acute ethanol treatment on NCCIT cells and NCCIT cell-derived embryoid bodies (EBs). Toxicol. In Vitro 24, 1696–1704 (2010).
Shevchenko, A. et al. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 93, 14440–14445 (1996).
Li, C.J., Li, R.W., Wang, Y.H. & Elsasser, T.H. Pathway analysis identifies perturbation of genetic networks induced by butyrate in a bovine kidney epithelial cell line. Funct. Integr. Genomics 7, 193–205 (2007).
Arzoine, L., Zilberberg, N., Ben-Romano, R. & Shoshan-Barmatz, V. Voltage-dependent anion channel 1-based peptides interact with hexokinase to prevent its anti-apoptotic activity. J. Biol. Chem. 284, 3946–3955 (2009).
Abu-Hamad, S. et al. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of antiapoptotic proteins. J. Cell. Sci. 2009; 122, 1906–1916 (2009).
Ito, M. et al. JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway. Mol. Cell. Biol. 19, 7539–7548 (1999).
Sabapathy, K. et al. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell 15, 713–725 (2004).
Xia, Z., Dickens, M., Raingeaud, J., Davis, R.J. & Greenberg, M.E. Opposing effects of ERK and JNKp38 MAP kinases on apoptosis. Science 270, 1326–1331 (1995).
Almeida, E.A. et al. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J. Cell Biol. 149, 741–754 (2000).
Papait, R. et al. Np95 is implicated in pericentromeric heterochromatin replication and in major satellite silencing. Mol. Biol. Cell 18, 1098–1106 (2007).
Papait, R. et al. The PHD domain of Np95 (mUHRF1) is involved in large-scale reorganization of pericentromeric heterochromatin. Mol. Biol. Cell 19, 3554–3563 (2008).
Garrido, C. et al. Heat shock proteins 27 and 70: antiapoptotic proteins with tumorigenic properties. Cell Cycle 5, 2592–2601 (2006).
Wang, X.Y. et al. Characterization of native interaction of hsp110 with hsp25 and hsc70. FEBS Lett. 465, 98–102 (2000).
Desai, A. & Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 (1997).
Desai, A. & Mitchison, T.J. A new role for motor proteins as couplers to depolymerizing microtubules. J. Cell Biol. 128, 1–4 (1995).
Berridge, M.J. & Irvine, R.F. Inositol phosphates and cell signalling. Nature 341, 197–205 (1989).
Steger, D.J., Haswell, E.S., Miller, A.L., Wente, S.R. & O’Shea, E.K. Regulation of chromatin remodeling by inositol polyphosphates. Science 299, 114–116 (2003).
Tsui, M.M. & York, J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzyme Regul. 50, 324–337 (2010).
Goldberg, A.L. & Rock, K.L. Proteolysis, proteasomes and antigen presentation. Nature 357, 375–379 (1992).
Selkoe, D.J. et al. Folding proteins in fatal ways. Nature 426, 900–904 (2003).
Liu, L., Mo, J., Rodriguez-Belmonte, E.M. & Lee, M.Y. Identification of a fourth subunit of mammalian DNA polymerase delta. J. Biol. Chem. 275, 18739–18744 (2000).
Yang, C.L. et al. Molecular cloning of the cDNA for the catalytic subunit of human DNA polymerase delta. Nucleic Acids Res. 20, 735–745 (1992).
Zeng, X.R., Hao, H., Jiang, Y. & Lee, M.Y. Regulation of human DNA polymerase delta during the cell cycle. J. Biol. Chem. 269, 24027–24033 (1994).
Wu, S.M. et al. Characterization of the p125 subunit of human DNA polymerase delta and its deletion mutants. Interaction with cyclin-dependent kinase-cyclins. J. Biol. Chem. 273, 9561–9569 (1998).
Ezaki, J., Takeda-Ezaki, M., Oda, K. & Kominami, E. Characterization of endopeptidase activity of tripeptidyl peptidase-I/CLN2 protein which is deficient in classical late infantile neuronal ceroid lipofuscinosis. Biochem. Biophys. Res. Commun. 268, 904–908 (2000).
Lin, L., Sohar, I., Lackland, H. & Lobel, P. The human CLN2 protein/tripeptidyl-peptidase I is a serine protease that autoactivates at acidic pH. J. Biol. Chem. 276, 2249–2255 (2001).
Golabek, A.A. et al. Biosynthesis, glycosylation, and enzymatic processing in vivo of human tripeptidyl-peptidase I. J. Biol. Chem. 278, 7135–7145 (2003).
Schwaller, M., Wilkinson, B. & Gilbert, H.F. Reduction-reoxidation cycles contribute to catalysis of disulfide isomerization by protein-disulfide isomerase. J. Biol. Chem. 278, 7154–7159 (2003).
Sideraki, V. & Gilbert, H.F. Mechanism of the antichaperone activity of protein disulfide isomerase: facilitated assembly of large, insoluble aggregates of denatured lysozyme and PDI. Biochemistry 39, 1180–1188 (2000).
Puig, A. & Gilbert, H.F. Protein disulfide isomerase exhibits chaperone and anti-chaperone activity in the oxidative refolding of lysozyme. J. Biol. Chem. 269, 7764–7771 (1994).
Dabiri, G.A., Young, C.L., Rosenbloom, J. & Southwick, F.S. Molecular cloning of human macrophage capping protein cDNA. A unique member of the gelsolin/villin family expressed primarily in macrophages. J. Biol. Chem. 267, 16545–16552 (1992).
Sun, H.Q., Kwiatkowska, K., Wooten, D.C. & Yin, H.L. Effects of CapG overexpression on agonistinduced motility and second messenger generation. J. Cell Biol. 129, 147–156 (1995).
Lamkanfi, M., Declercq, W., Kalai, M., Saelens, X. & Vandenabeele, P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 9, 358–361 (2002).
Lamkanfi, M., Festjens, N., Declercq, W., Vanden Berghe, T. & Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 14, 44–55 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Das, A., Das, N.D., Park, J.H. et al. Identification of survival factors in LPS-stimulated anthrax lethal toxin tolerant RAW 264.7 cells through proteomic approach. BioChip J 7, 75–84 (2013). https://doi.org/10.1007/s13206-013-7112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-013-7112-0