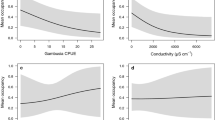
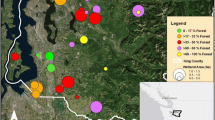
Abstract
Fish introduced into wetlands can impact amphibian populations through predation on eggs and larvae. While relationships among hydroperiod, habitat complexity and predation on amphibian larvae have been examined in relatively natural freshwater ecosystems, they have not been explicitly considered in urban landscapes. We examined these relationships in 64 urban wetlands in southern Australia using non-native fish and aquatic invertebrates as predators. Larvae of three out of six frog species detected during our study were captured in wetlands containing fish. With other variables held constant, the mean number of tree frog (Litoria spp.) larvae in the wetland with the highest abundance of predatory fish was predicted to be only 0.8–3.2 % of the number of larvae in a fishless wetland. We also found a negative relationship between predatory invertebrates and larval abundance. The abundance of tree frog larvae was greatest in ephemeral wetlands where predatory fish were generally absent. Our results suggest that traditional models of amphibian distribution along pond-permanence gradients may not be applicable in urban ecosystems due to modified hydrology favoring permanent wetlands. To conserve amphibians in urban areas, we recommend draining wetlands periodically to remove exotic fish, and conserving or restoring ephemeral wetlands.




Similar content being viewed by others
References
Adams MJ (1999) Correlated factors in amphibian decline: exotic species and habitat change in western Washington. Journal of Wildlife Management 63:1162–1171
Adams MJ, Richter KO, Leonard WP (1997) Surveying and monitoring amphibians using aquatic funnel traps. In: Olson DH, Leonard WP, Bury RB (eds) Sampling Amphibians in Lentic Habitats. Northwest Fauna Number 4, Society for Northwestern Vertebrate Biology, Olympia, Washington, pp 47–54
Anderson DR (2008) Model based inference in the life sciences: a primer on evidence. Springer Science + Business Media, New York
Anstis M (2002) Tadpoles of South-eastern Australia: a guide with keys. Reed New Holland, Sydney
Australian Bureau of Statistics (2010) Year Book Australia, 2009–10, No. 91. Australian Bureau of Statistics, Canberra, Australia
Babbitt KJ, Tanner GW (1997) Effects of cover and predator identity on predation of Hyla squirella tadpoles. Journal of Herpetology 31:128–130
Babbitt KJ, Baber MJ, Tarr TL (2003) Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Canadian Journal of Zoology 81:1539–1552
Babbitt KJ, Baber MJ, Childers DL, Hocking D (2009) Influence of agricultural upland habitat type on larval anuran assemblages in seasonally inundated wetlands. Wetlands 29:294–301
Baber MJ, Babbitt KJ (2004) Influence of habitat complexity on predator–prey interactions between the fish (Gambusia holbrooki) and tadpoles of Hyla squirella and Gastrophryne carolinensis. Copeia 2004:173–177
Both C, Cechin SZ, Melo AS, Hartz SM (2011) What controls tadpole richness and guild composition in ponds in subtropical grasslands? Austral Ecology 36:530–536
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27:325–349
Brönmark C, Edenhamn P (1994) Does the presence of fish affect the distribution of tree frogs (Hyla arborea)? Conservation Biology 8:841–845
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics 7:434–455
Casterlin ME, Reynolds WW (1977) Aspects of habitat selection in the mosquitofish Gambusia affinis. Hydrobiologia 55:125–127
Clarke KR, Gorley RN (2001) PRIMER v5: user manual/tutorial. PRIMER-E, Plymouth
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Diversity and Distributions 9:89–98
Copp GH, Wesley KJ, Vilizzi L (2005) Pathways of ornamental and aquarium fish introductions into urban ponds of Epping Forest (London, England): the human vector. Journal of Applied Ichthyology 21:263–274
Cumming G, Finch S (2005) Inference by eye: confidence intervals and how to read pictures of data. American Psychologist 60:170–180
Gamradt SC, Kats LB (1996) Effect of introduced crayfish and mosquitofish on California newts. Conservation Biology 10:1155–1162
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Statistical Science 7:457–472
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biological Conservation 141:2432–2449
Hamer AJ, Parris KM (2011) Local and landscape determinants of amphibian communities in urban ponds. Ecological Applications 21:378–390
Hamer AJ, Lane SJ, Mahony MJ (2002) The role of introduced mosquitofish (Gambusia holbrooki) in excluding the native green and golden bell frog (Litoria aurea) from original habitats in south-eastern Australia. Oecologia 132:445–452
Harris RN (1999) The anuran tadpole. Evolution and maintenance. In: McDiarmid RW, Altig R (eds) Tadpoles. The biology of anuran larvae. The University of Chicago Press, Chicago, pp 279–294
Hartel T, Nemes S, Cogălniceanu D, Öllerer K, Schweiger O, Moga CI, Demeter L (2007) The effect of fish and aquatic habitat complexity on amphibians. Hydrobiologia 583:173–182
Hero JM, Littlejohn M, Marantelli G (1991) Frogwatch field guide to Victorian frogs. Department of Conservation and Environment, East Melbourne
Hero JM, Gascon C, Magnusson WE (1998) Direct and indirect effects of predation on tadpole community structure in the Amazon rainforest. Australian Journal of Ecology 23:474–482
Heyer WR, McDiarmid RW, Weigmann DL (1975) Tadpoles, predation and pond habitats in the tropics. Biotropica 7:100–111
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Diversity and Distributions 9:99–110
Kentula ME, Gwin SE, Pierson SM (2004) Tracking changes in wetlands with urbanization: sixteen years of experience in Portland, Oregon, USA. Wetlands 24:734–743
Lee SY, Dunn RJK, Young RA, Connolly RM, Dale PER, Dehayr R, Lemckert CJ, McKinnon S, Powell B, Teasdale PR, Welsh DT (2006) Impact of urbanization on coastal wetland structure and function. Austral Ecology 31:149–163
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecology Letters 8:1235–1246
McCarthy MA (2007) Bayesian methods for ecology. Cambridge University Press, Cambridge
McDonnell MJ, Holland K (2008) Biodiversity. In: Newton PW (ed) Transitions: transitioning to resilient cities. CSIRO, Melbourne, pp 253–266
Morgan LA, Buttemer WA (1996) Predation by the non-native fish Gambusia holbrooki on small Litoria aurea and L. dentata tadpoles. Australian Zoologist 30:143–149
Parris KM (2006) Urban amphibian assemblages as metacommunities. Journal of Animal Ecology 75:757–764
Pearl CA, Adams MJ, Leuthold N, Bury RB (2005) Amphibian occurrence and aquatic invaders in a changing landscape: implications for wetland mitigation in the Willamette Valley, Oregon, USA. Wetlands 25:76–88
Pechmann JHK, Scott DE, Gibbons JW, Semlitsch RD (1989) Influence of wetland hydroperiod on diversity and abundance of metamorphosing juvenile amphibians. Wetlands Ecology and Management 1:3–11
Peterson AG, Bull MC, Wheeler LM (1992) Habitat choice and predator avoidance in tadpoles. Journal of Herpetology 26:142–146
Pyke GH (2005) A review of the biology of Gambusia affinis and G. holbrooki. Reviews in Fish Biology and Fisheries 15:339–365
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annual Review of Ecology, Evolution and Systematics 39:171–191
Pyke GH, White AW (2000) Factors influencing predation on eggs and tadpoles of the endangered green and golden bell frog Litoria aurea by the introduced plague minnow Gambusia holbrooki. Australian Zoologist 31:496–505
Richter KO (1995) A simple aquatic funnel trap and its application to wetland amphibian monitoring. Herpetological Review 26:90–91
Riley SPD, Busteed GT, Kats LB, Vandergon TL, Lee LFS, Dagit RG, Kerby JL, Fisher RN, Sauvajot RM (2005) Effects of urbanization on the distribution and abundance of amphibians and invasive species in southern California streams. Conservation Biology 19:1894–1907
Rubbo MJ, Kiesecker JM (2005) Amphibian breeding distribution in an urbanized landscape. Conservation Biology 19:504–511
Semlitsch RD, Scott DE, Pechmann JHK, Gibbons JW (1996) Structure and dynamics of an amphibian community: evidence from a 16-year study of a natural pond. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic, San Diego, pp 217–248
Shaffer HB, Alford RA, Woodward BD, Richards SJ, Altig RG, Gascon C (1994) Quantitative sampling of amphibian larvae. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and monitoring biological diversity. Standard methods for amphibians. Smithsonian Institution Press, Washington, DC, pp 130–141
Shulse CD, Semlitsch RD, Trauth KM, Williams AD (2010) Influences of design and landscape placement parameters on amphibian abundance in constructed wetlands. Wetlands 30:915–928
Shulse CD, Semlitsch RD, Trauth KM, Gardner JE (2012) Testing wetland features to increase amphibian reproductive success and species richness for mitigation and restoration. Ecological Applications 22:1675–1688
Skelly DK (1996) Pond drying, predators, and the distribution of Pseudacris tadpoles. Copeia 3:599–605
Snodgrass JW, Bryan AL Jr, Burger J (2000a) Development of expectations of larval amphibian assemblage structure in southeastern depression wetlands. Ecological Applications 10:1219–1229
Snodgrass JW, Komoroski MJ, Bryan AL Jr, Burger J (2000b) Relationships among isolated wetland size, hydroperiod, and amphibian species richness: implications for wetland regulations. Conservation Biology 14:414–419
Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002) Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B 64:583–639
Spiegelhalter D, Thomas A, Best N, Lunn D (2007) OpenBUGS user manual, version 3.0.2. MRC Biostatistics Unit, Cambridge
Stern H (2005) Climate. In: Brown-May A, Swain S (eds) The encyclopedia of Melbourne. Cambridge University Press, Melbourne, pp 147–154
Tarr TL, Babbitt KJ (2002) Effects of habitat complexity and predator identity on predation of Rana clamitans larvae. Amphibia-Reptilia 23:13–20
Tyre AJ, Tenhumberg B, Field SA, Niejalke D, Parris K, Possingham HP (2003) Improving precision and reducing bias in biological surveys: estimating false-negative error rates. Ecological Applications 13:1790–1801
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27:337–363
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Welsh AH, Cunningham RB, Donnelly CF, Lindenmayer DB (1996) Modelling the abundance of rare species: statistical models for counts with extra zeros. Ecological Modelling 88:297–308
Werner EE, Skelly DK, Relyea RA, Yurewicz KL (2007) Amphibian species richness across environmental gradients. Oikos 116:1697–1712
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wintle BA, Bardos DC (2006) Modeling species-habitat relationships with spatially autocorrelated observation data. Ecological Applications 16:1945–1958
Acknowledgments
We thank Sam Davis, Terry Coates, Andrew Gay, Andrew Smith and Colin Walker for facilitating access to wetland sites. Geoff Heard and Eliza Poole assisted with fieldwork. Michael Smith provided advice on trap design. We also thank Marion Anstis for assistance with tadpole identification, and Tara Martin, Michael McCarthy and Joslin Moore for assistance with statistical modeling. The Baker Foundation and National Environmental Research Program (Research Hub for Environmental Decisions) provided generous support for this research. This study was approved by the University of Melbourne Animal Ethics Committee (register no. 0706488). Fieldwork was conducted under research permit no. 10004319 issued by the Victorian Department of Sustainability and Environment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Spearman correlation coefficients (Spearman’s rho, ρ) among predator and habitat variables (PDF 61 kb)
Online Resource 2
Summary of aquatic sampling at 64 urban wetlands in the Greater Melbourne area, Australia (PDF 9 kb)
Online Resource 3
The mean CPUE of predatory fish (mosquitofish and redfin perch) and the larvae of six frog species according to hydroperiod at 64 wetlands in the Greater Melbourne area, Australia, 2007–2008. CPUE data were log(x+1)-transformed to reduce the influence of large values and to aid in interpretation. Permanent wetlands had a hydroperiod score = 1; ephemeral wetlands had a score <1. (PDF 15 kb)
Online Resource 4
Proportion of predatory invertebrate taxa captured at the 64 wetlands (PDF 8 kb)
Rights and permissions
About this article
Cite this article
Hamer, A.J., Parris, K.M. Predation Modifies Larval Amphibian Communities in Urban Wetlands. Wetlands 33, 641–652 (2013). https://doi.org/10.1007/s13157-013-0420-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0420-2