Abstract
Background
The combined spinal-epidural (CSE) technique for relief of labour pain offers both rapid onset and superior first-stage analgesia. Nevertheless, the known increased risk for early profound fetal bradycardia (EPFB) following CSE continues to be a concern that often limits its use. The purpose of this study was to determine if giving prophylactic intravenous ephedrine at the time of CSE administration would reduce EPFB.
Methods
We conducted this clinical trial at a large community hospital and enrolled healthy patients requesting epidural analgesia for labour. Patients were randomly assigned to receive either normal saline placebo or ephedrine 10 mg iv at the time of CSE. The primary outcome of EPFB (defined as bradycardia < 90 beats·min−1 for > two minutes and occurring within the first 30 min after CSE) was compared between groups. The secondary outcomes included the incidence of urgent cesarean delivery, the requirement for additional doses of ephedrine, maternal blood pressure, uterine hypertonus and tachysystole, and abnormal fetal heart rate (FHR) patterns before and after CSE.
Results
There were 299 women randomized to the ephedrine (EPH) group and 297 randomized to the normal saline placebo (NS) group. There was no difference between groups in the incidence of EPFB (2.7% EPH group vs 4.7% NS group; relative risk, 0.57; 95% confidence interval, 0.24 to 1.33; P = 0.184). There was also no difference between groups in the incidence of urgent cesarean delivery, uterine hypertonus, uterine tachysystole, and abnormal FHR patterns.
Conclusions
We conclude that prophylactic intravenous ephedrine administration at the time of CSE during labour was ineffective at reducing the risk for EPFB associated with CSE. Nevertheless, a lower than expected rate of EPFB resulted in the trial being underpowered. This trial was registered at ClinicalTrials.gov, identifier: NCT02062801.
Résumé
Contexte
La technique rachidienne et péridurale combinée (RPC) pour soulager la douleur lors de l’accouchement procure à la fois un court délai d’action et une analgésie supérieure pour la première étape du travail obstétrical. Cependant, le risque accru connu de bradycardie fœtale profonde précoce (BFPP) après une RPC est une inquiétude qui contribue bien souvent à freiner son utilisation. L’objectif de cette étude était de déterminer si l’administration prophylactique d’éphédrine intraveineuse au moment de l’administration de la RPC réduisait la BFPP.
Méthode
Nous avons réalisé cette étude clinique dans un hôpital communautaire d’envergure et enrôlé des patientes ayant demandé une analgésie péridurale pour le travail obstétrical. Les patientes ont été aléatoirement réparties en deux groupes et ont reçu soit un placebo de sérum physiologique ou 10 mg iv d’éphédrine au moment de la RPC. Nous avons comparé notre critère d’évaluation principal, la BFPP (définie comme une bradycardie < 90 battements·min−1 pour > 2 minutes et survenant au cours des 30 premières minutes après la RPC), entre les deux groupes. Les critères d’évaluation secondaires comprenaient l’incidence d’accouchement urgent par césarienne, la demande de doses supplémentaires d’éphédrine, la pression artérielle maternelle, l’hypertonie et la tachysystolie utérines, et des rythmes de fréquence cardiaque fœtale (FCF) anormaux avant et après la RPC.
Résultats
Au total, le groupe éphédrine (EPH) comptait 299 femmes et le groupe placebo de sérum physiologique (NS) 297 femmes. Aucune différence entre les groupes n’a été observée en matière d’incidence de BFPP (2,7 % dans le groupe EPH vs 4,7 % dans le groupe NS; risque relatif, 0,57; intervalle de confiance 95 %, 0,24 à 1,33; P = 0,184). Aucune différence n’a été observée entre les groupes en matière d’incidence d’accouchement urgent par césarienne, d’hypertonie utérine, de tachysystolie utérine et de rythmes de FCF anormaux.
Conclusion
Nous concluons que l’administration prophylactique d’éphédrine intraveineuse au moment d’une RPC pendant le travail obstétrical est inefficace pour réduire le risque de BFPP associée à la RPC. Néanmoins, le taux de BFPP dans l’essai a été inférieur à ce qui était attendu, ce qui a entraîné un manque de puissance de l’étude. L’étude a été enregistrée sur le site ClinicalTrials. gov sous le numéro NCT02062801.
Similar content being viewed by others
The combined spinal-epidural (CSE) technique for labour pain relief has become more popular because of its rapid onset, superior first-stage analgesia, and the requirement for fewer supplemental doses compared with a standard epidural technique.1,2 Despite these benefits, an increased risk for fetal bradycardia following CSE is a concern and is one of the reasons why some anesthesiologists are hesitant to use CSE more frequently.3,4 The causes of fetal bradycardia in this setting are uncertain but may be associated with a number of factors, including a reduction in uteroplacental blood flow from the sympathetic block induced by CSE; reductions in uterine blood flow secondary to uterine overactivity, which can be caused by rapid onset analgesia leading to a catecholamine imbalance; and/or rapid decent of the neonate’s head causing a fetal vagal response.5,6 Other possible causes of profound fetal bradycardia, not associated with CSE, include compression of the umbilical cord and, in rare cases, maternal seizures.
Since abrupt onset maternal hypotension and a subsequent reduction in uteroplacental blood flow are thought to contribute to fetal bradycardia,7 we focused this study on the use of intravenous ephedrine which has been used to prevent abrupt hypotension shortly after initiating CSE.2,8,9 The definition of profound fetal bradycardia differs across specialty organizations, but most define it as an abrupt decrease in fetal heart rate (FHR) to levels more than 15 beats·min−1 below baseline that last at least 60-90 sec. Profound fetal bradycardia is a risk to the fetus if it lasts for more than three minutes.7,10 Our study defines profound fetal bradycardia somewhat more stringently than current guidelines to reflect the timing of occurrence, i.e., early profound fetal bradycardia (EPFB) is defined as a prolonged FHR deceleration to less than 90 beats·min−1 for at least two minutes in the 30-min time frame immediately following CSE. Furthermore, in our own practice, this definition of EPFB allows time for conservative treatment to be implemented before it becomes a trigger for activating an obstetrical rapid response team.
Although studies have reported the prophylactic use of intravenous ephedrine during CSE administration with the goal of reducing the risk of fetal bradycardia8,9 and the practice is routine at our own hospital, there is currently a lack of clinical trial evidence to guide this practice. Therefore, the purpose of the current study was to determine whether a prophylactic dose of intravenous ephedrine 10 mg given at the time of CSE reduces the incidence of EPFB and other potential adverse outcomes when compared with placebo. Our hypothesis was that prophylactic intravenous ephedrine would decrease the incidence of EPFB. Secondary outcomes included the incidence of urgent cesarean delivery, the requirement for additional doses of ephedrine, maternal blood pressure (MBP), uterine hypertonus and tachysystole, and FHR patterns before and after CSE.
Methods
The Institutional Review Board at our hospital gave approval for this prospective placebo-controlled double-blind randomized study in December 2011 (Sharp HealthCare’s IRB#111199).
The study sample included healthy females in labour requesting epidural analgesia at the labour and delivery unit of Sharp Mary Birch Hospital for Women and Newborns in San Diego, California from January 24, 2012 to April 12, 2013. The inclusion criteria for participation comprised the ability to speak English, American Society of Anesthesiologists physical status I-III, and an uncomplicated term labour. The exclusion criteria included a gestational age < 37 weeks, a malpresentation, previous cesarean delivery, multiple gestation, chronic hypertension, preeclampsia, and heart conditions for which ephedrine use is contraindicated (e.g., coronary heart disease, preexisting cardiac dysrhythmias, or patients with a history of Wolff-Parkinson-White syndrome and other causes of supraventricular tachycardia). Eligible women were approached for study participation after admission to the hospital’s labour and delivery unit. Voluntary written consent was obtained from participants between admission to the hospital and a request for epidural analgesia.
At the time of CSE, the subjects were randomly assigned to receive either ephedrine 10 mg iv or a saline placebo injected over a five- to ten-second period. A statistician created randomization tables that were kept by a pharmacist who was unblinded to group assignment. We used an SPSS® version 21.0 (IBM Corp. Armonk, NY, USA) macro for random selection (using a uniform distribution) of n = 10 subjects from each block (each block had 20 subjects) for treatment allocation. A pharmacist prepared 1 mL of ephedrine and 1 mL of saline placebo in labeled syringes of equal size, and the study drugs were placed in separate sealed opaque envelopes each labeled with a sequential study number. The study subjects were then assigned consecutively to the next study number. The envelopes containing the study drug were stored in a password-protected medication dispensing system. The patient, anesthesiologist, bedside nurse, and obstetrician were blinded to group assignment. The anesthesiologist opened the envelope and then handed the syringe to the bedside nurse who administered the drug at the instruction of the anesthesiologist.
All patients received a 500 mL bolus of Ringer’s Lactate solution prior to the CSE. Patients received the CSE in a sitting position at the L2-3 or L3-4 interspace. A needle-through-needle technique was used exclusively. This involved passing a 26G Gertie Marx® needle (International Medical Development, Park City, UT, USA) through a previously sited epidural needle in order to obtain flow of cerebrospinal fluid. The subarachnoid and epidural drug doses were standardized with each patient receiving subarachnoid bupivacaine 3.125 mg plus fentanyl 5 µg as the initial spinal dose (i.e., 0.125% bupivacaine 2.5 mL plus fentanyl 2 µg·mL−1), well within the established range for intrathecal dosing.4,11 The bedside nurse was instructed to give the study drug intravenously as a bolus within 60 sec of administration of the spinal dose. After the epidural catheter was securely placed, all patients were positioned in a left lateral position for at least 30 min after CSE placement, and the continuous FHR and uterine contraction monitors were repositioned. After 15-20 min, the patients received patient-controlled epidural analgesia for maintenance. An automated blood pressure cuff was used to measure blood pressure from the upper arm every two minutes for ten minutes, every five minutes for the next 20 min, and then every 30 min after CSE. The bedside nurses were instructed to treat hypotension promptly with intravenous ephedrine supplements; this involved treatment of systolic blood pressure < 90 mmHg with ephedrine 10 mg iv up to a total dose of 30 mg and an intravenous bolus of Ringer’s Lactate solution 250 mL. The nurses were also instructed to call the anesthesiologist for all cases of EPFB or sustained uterine contraction. For sustained uterine contraction or symptomatic uterine tachysystole, the anesthesiologist administered two puffs of metered sublingual nitroglycerine spray or, if not available, the nurse administered a subcutaneous dose of terbutaline per hospital protocol.
Demographic data were collected on data entry forms and/or extracted from the hospital’s data warehouse. The anesthesiologist documented MBP, FHR, and maternal pain scores immediately prior to induction of CSE. The patient was instructed to provide a numeric score of pain during a contraction (0 = no pain and 10 = the worst pain possible). We also documented the time of intrathecal injection, the time of administration of the study drug, the total dose of intravenous fentanyl received, and the time from the last dose of fentanyl to insertion of CSE. Uterine contractions were recorded continuously ten minutes before and 30 min after starting the CSE. Blood pressure readings were extracted from the electronic health record, and nurses blinded to group assignment conducted post hoc assessments of the FHR strips. The nurses were specially trained in FHR monitoring and were supervised by a maternal/child clinical nurse specialist. The baseline FHR, decelerations (early, late, or variable), and the presence of uterine tachysystole were determined using standardized definitions developed by the National Institute of Child Health and Human Development.10 Sustained tetanic uterine contraction (TUC) was defined as a contraction lasting for more than two minutes, and uterine tachysystole was defined as more than five contractions in ten minutes averaged over a 30-min window.
Statistical analysis
Previous research conducted at our hospital identified an EPFB incidence of 8.5%.2 We anticipated an approximate 50% reduction in primary outcome based on our own anecdotal data of an expected ephedrine effect. Accordingly, we used the Power and Precision 2.1 macro on SPSS version 21.0 to run a one-trial simulation using z test of proportions and prospectively calculated the sample size needed to detect a 4% absolute difference in outcome (i.e., from 8.5% to 4.5%) with an alpha of .05 with a power of 0.8. The power analysis determined that 298 patients per group would suffice.
Extensive screening of the data was conducted to assure the accuracy of the data fields and to identify missing fields. Data are presented as median, mean, and standard deviation. For our primary outcome, a one-way analysis of variance (ANOVA) was used to compare between-group differences. For the secondary outcome data, one-way ANOVA was used to compare between-group differences. A Chi square test of independence was conducted for categorical demographic and secondary outcome nominal variables, and a mixed ANOVA was conducted to assess the impact of grouping (ephedrine vs placebo) and time (pre-CSE vs 30 min post CSE) on FHR and MBP. Statistical analysis was conducted using SPSS version 21.0 and, when warranted, Stata® version 11.0 (StataCorp LP, College Station, TX, USA). All reported P values are two sided.
Results
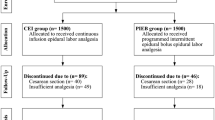
The study included 299 subjects in the ephedrine (EPH) group and 297 subjects in the placebo (NS) group (Fig. 1). Baseline patient variables are described in Table 1. The groups were similar with respect to pain scores at the time of CSE, the use of fentanyl prior to CSE, and the time from last dose of fentanyl to injection of the study drug (Table 1).
There was no difference between the groups for the primary outcome, i.e., the incidence of EPFB (4.7% NS vs 2.7% EPH; relative risk [RR], 0.57; 95% confidence interval [CI], 0.24 to 1.33; P = 0.184) (Table 2). As for the secondary outcomes, one woman in the EPH group and two in the NS group required an emergency cesarean delivery within 30 min of receiving CSE. All three cases were related to the occurrence of EPFB after CSE.
The incidence of the supplemental ephedrine use was higher for the NS group than for the EPH group (29% NS vs 13.4% EPH; RR, 2.16; 95% CI, 1.54 to 3.04; P < 0.001). In addition, there was a significant difference between the mean (SD) supplemental ephedrine dose in the EPH group [1.8 (5.0) mg] vs the NS group [3.9 (7.0) mg] (P < .001) (Table 2 and Fig. 2). Maternal systolic blood pressure at 30 min post CSE was significantly different between groups, with the EPH group having higher mean (SD) systolic blood pressure than the NS group [113 (11) mmHg vs 107 (10) mmHg, respectively; P < 0.05] (Table 3).
The overall incidence of uterine tachysystole after CSE was 3.5%, and there was no difference between groups (3.4% EPH group vs 3.7% NS group; P = 0.806). The overall incidence of sustained uterine contraction was 19.5%, but there was also no difference between groups (19.4% EPH group vs 19.5% NS group; P = 0.968). The majority of TUC (91.4% EPH group vs 89.7% NS group; P = 0.968) and uterine tachysystole (90% EPH group vs 81.8% NS group; P = 0.806) was not associated with EPFB (Table 2). Nevertheless, women who exhibited either TUC or uterine tachysystole had a 10.2% incidence of bradycardia compared with a 1.9% incidence in women who did not exhibit TUC or tachysystole (odds ratio, 5.8; 95% CI, 2.4 to 13.8; P = 0.009).
For FHR, the blinded evaluators saw no difference in any type of deceleration they assessed either before or after CSE (Table 3). In addition, there was no effect of time (P = 0.20), with both groups showing no significant changes in FHR over time (Table 3).
Discussion
We were unable to show any effect of prophylactic intravenous ephedrine on the incidence of EPFB after CSE. This was despite studying a much larger population than prior studies and using a vigorous prospective randomized blinded study design.
The results do not align with previous studies that have shown a reduction in fetal bradycardia when ephedrine is given at the time of epidural analgesia,8,9 although direct comparison of results is difficult as these studies used different analgesic methods and endpoints as well as varying doses and routes of administration of ephedrine. Kreiser et al.8 found that a continuous intravenous infusion of ephedrine 20 mg for one hour reduced the rate of major FHR changes appearing up to 25 min after epidural analgesia. Cleary-Goldman et al.9 evaluated prophylactic intramuscular ephedrine 25 mg vs placebo after CSE and found that intramuscular ephedrine decreased the incidence of maternal hypotension and late FHR decelerations for one hour after administration but increased the incidence of fetal tachycardia. Nevertheless, FHR reactivity was improved.
There were no differences in significant adverse outcomes between groups. The overall incidence of uterine tachysystole after CSE was 3.5%, and the incidence of sustained uterine contraction was 19.5%. Furthermore, the overwhelming majority of these occurrences were not associated with EPFB (Table 2). For patients with EPFB, however, 63% in the EPH group also had TUC compared with 43% in the NS group. These results contradict a study that examined the relationship between uterine tone and FHR changes after neuraxial blockade.12 In that study, 42% of patients with CSE had elevated uterine tone and an odds ratio of 18.62 for bradycardia or prolonged decelerations when elevation of uterine tone was present. Our study found the opposite, i.e., 91% of patients with TUC did not have EPFB, and 50% of those with EPFB had TUC. This discrepancy might be explained by different methods of measuring uterine tone, internal vs external monitors, and different definitions of fetal bradycardia among studies
The incidence of fetal bradycardia is known to be greater with higher doses of intrathecal opioid, perhaps due to an increase in resting uterine tone.13,14 Rapid pain relief induces a significant decrease in maternal circulating catecholamines, especially epinephrine. The tocolytic effect of epinephrine is reduced and norepinephrine’s uterotonic effect is enhanced. The resulting imbalance may lead to uterine hypertonus, a fall in uteroplacental perfusion, and subsequent fetal bradycardia. We used a lower initial dose of spinal fentanyl (5 µg) than most studies in an attempt to reduce itching associated with its use. Hence, our spinal dose of bupivacaine was adjusted up to 3.125 mg from 2.5 mg in order to compensate for the smaller fentanyl dose (5 µg vs 10-25 µg). Some might argue that a larger dose of bupivacaine could create higher rates of hypotension; however, the dose was the same in both treatment arms and is one commonly used at our hospital.
There was a significant difference in MBP between groups. Although the groups had similar MBP at the time of CSE, the EPH group had higher systolic blood pressure 30 min post CSE than the NS group. This result confirms prior evidence showing that prophylactic ephedrine reduces the risk for maternal hypotension,15 and since our rates of EPFB did not differ between groups, this suggests that maternal hypotension alone may not be a major contributor to the onset of EPFB. Indeed, our study has shown a fivefold increase in the incidence of EPFB when uterine tachysystole or uterine sustained contractions occur, indicating that uterine tone plays a role.
There were no significant differences between groups in terms of abnormal FHR patterns. The EPH group had a slightly higher but clinically insignificant FHR 30 min post CSE than the NS group, which showed no changes in FHR. This study did not confirm prior evidence of the benefit of ephedrine for reducing the incidence of decreased FHR,9 but it showed that there was no corresponding incidence of fetal tachycardia associated with prophylactic ephedrine administration.
Our data on abnormal FHR patterns is interesting in that it shows the incidence of early and late FHR decelerations as well as variable decelerations after CSE. Ephedrine administration at the time of CSE has no impact on the incidence of these abnormal patterns. Abnormalities in FHR also occurred in 25% of patients prior to CSE, but most were not a clinical concern. These findings are similar to those from a recent study that looked at FHR decelerations both before and after CSE and epidural analgesia.16 They showed that fetal decelerations occurred in 3.2-14.5% of parturients prior to CSE and that the incidence of these decelerations increases after initiating analgesia. They also concluded that these changes in FHR did not affect neonatal outcomes.
The present study also showed that the use of supplementary ephedrine to treat hypotension post CSE was significantly reduced, both in incidence and total dose, in the EPH group compared with the NS group. Using prophylactic ephedrine to reduce the need to treat hypotension may save time for clinicians, reduce episodes that may cause the mother additional anxiety or worry, and reduce the potential for dosing errors and adverse outcomes.
Despite its strengths in terms of patient numbers and blinding the clinicians involved, there were some limitations to this study. The rate of EPFB was found to be much lower in this study than in an earlier study conducted at the same hospital; that study identified an EPFB rate of 8.5% as a secondary outcome.2 The earlier study’s EPFB rate was used to calculate the sample size for this study. A post hoc power analysis using the EPFB rate (4.7%) of the earlier study’s control group determined that a sample size of more than 1,000 patients per arm would be needed to detect a statistically significant difference, thus our study was underpowered. Considering these facts, the lack of significance for the primary outcome may be attributed to type II error (falsely accepting the null hypothesis).
Overall, we conclude that prophylactic intravenous ephedrine administration at the time of CSE during labour appears to be an ineffective means of reducing the risk for EPFB associated with CSE. Nevertheless, the underpowered nature of our study limits definitive conclusions about the efficacy of prophylactic ephedrine in this setting, and a much larger study would be required to determine any impact. That said, if the effect of ephedrine on EPFB is so small, it might not be of clinical significance.
References
Cook TM. Combined spinal-epidural techniques. Anaesthesia 2000; 55: 42-64.
Gambling D, Berkowitz J, Farrell TR, Pue A, Shay D. A randomized controlled comparison of epidural analgesia and combined spinal-epidural analgesia in a private practice setting: pain scores during first and second stages of labor and at delivery. Anesth Analg 2013; 116: 636-43.
Preston R. The role of combined spinal epidural analgesia for labour: is there still a question? Can J Anesth 2007; 54: 9-14.
Wong CA. Epidural and spinal analgesia/anesthesia for labor and vaginal delivery. In: Chestnut DH, et al., editors. Obstetric Anesthesia: Principles and Practice. 4ª ed. Philadelphia: Mosby Elsevier; 2009. p. 429-92.
Friedlander JD, Fox HE, Cain CF, Dominguez CL, Smiley RM. Fetal bradycardia and uterine hyperactivity following subarachnoid administration of fentanyl during labor: case report. Reg Anesth Pain Med 1997; 22: 378-81.
Nicolet J, Miller A, Kaufman I, Guertin MC, Deschamps A. Maternal factors implicated in fetal bradycardia after combined spinal epidural for labour pain. Eur J Anaesthesiol 2008; 25: 721-5.
Liston R, Sawchuck D, Young D; Society of Obstetrics and Gynaecolgists of Canada; British Columbia Perinatal Health Program. Fetal health surveillance: antepartum and intrapartum consensus guideline. J Obstet Gynaecol Can 2007; 29(9 Suppl 4): S3-56.
Kreiser D, Katorza E, Seidman DS, Etchin A, Schiff E. The effect of ephedrine on intrapartum fetal heart rate after epidural analgesia. Obstet Gynecol 2004; 104: 1277-81.
Cleary-Goldman J, Negron M, Scott J, et al. Prophylactic ephedrine and combined spinal epidural: maternal blood pressure and fetal heart rate patterns. Obstet Gynecol 2005; 106: 466-72.
Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update o definitions, interpretation, and research guidelines. Obstet Gynecol 2008; 112: 661-6.
Stocks GM, Hallworth SP, Fernando R, England AJ, Columb MO, Lyons G. Minimum local analgesic dose of intrathecal bupivacaine in labor and the effect of intrathecal fentanyl. Anesthesiology 2001; 94: 593-8.
Abrao KC, Francisco RP, Miyadahira S, Cicarelli DD, Zugaib M. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol 2009; 113: 41-7.
Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG 2002; 109: 274-81.
Clarke VT, Smiley RM, Finster M. Uterine hyperactivity after intrathecal injection of fentanyl for analgesia during labor: a cause of fetal bradycardia? Anesthesiology 1994; 81: 1083.
Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg 2000; 90: 1390-5.
Patel NP, El-Wahab N, Fernando R, et al. Fetal effects of combined spinal-epidural vs epidural labour analgesia: a prospective, randomized double-blind study. Anaesthesia 2014; 69: 458-67.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
David R. Gambling, Miriam Bender, Sue Faron, and Thomas R. Farrell helped design the study. Miriam Bender, Sue Faron, and Thomas R. Farrell helped collect the data. David R. Gambling, Miriam Bender, and Sue Faron helped analyze the data and write the manuscript. David R. Gambling helped conduct the study. Sue Faron helped educate the nursing staff for study participation. Dale Glaser was involved in the statistical analysis, power analysis, proposed analysis, and the results. Thomas R. Farrell helped recruit patients and educate the nursing staff about the study protocol, and he reviewed the manuscript. All authors reviewed the analysis of the data.
Rights and permissions
About this article
Cite this article
Gambling, D.R., Bender, M., Faron, S. et al. Prophylactic intravenous ephedrine to minimize fetal bradycardia after combined spinal-epidural labour analgesia: a randomized controlled study. Can J Anesth/J Can Anesth 62, 1201–1208 (2015). https://doi.org/10.1007/s12630-015-0450-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0450-8