Abstract
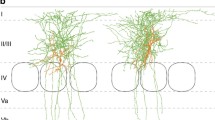
Parvalbumin (PV)-positive fast-spiking cells in the neocortex are known to generate gamma oscillations by mutual chemical and electrical connections. Recent findings suggest that this rhythm might be responsible for higher-order brain functions, and related to psychiatric disorders. To elucidate the precise structural rules of the connections of PV neurons, we first produced genetic tools. Using a lentiviral expression system, we developed neuron-specific promoters and a new reporter protein that labels the somatodendritic membrane of neurons. We applied the reporter protein to the generation of transgenic mice, and succeeded in visualizing the dendrites and cell bodies of PV neurons efficiently. Then we analyzed excitatory and inhibitory inputs to PV neurons in the primary somatosensory cortex using the mice. Corticocortical glutamatergic inputs were more frequently found on the distal dendrites than on the soma, whereas thalamocortical inputs did not differ between the proximal and distal portions. Corticocortical inhibitory inputs were more densely distributed on the soma than on the dendrites. We further investigated which types of neocortical GABAergic neurons preferred the PV soma over their dendrites. We revealed that the somatic and dendritic compartments principally received GABAergic inputs from vasoactive intestinal polypeptide (VIP)-positive and PV neurons, respectively. This compartmental organization suggests that PV neurons communicate with each other mainly via the dendrites, and that their activity is effectively controlled by the somatic inputs of VIP neurons. These findings provide new insights into the neuronal circuits involving PV neurons, and contribute to a better understanding of brain functions and mental disorders.







Similar content being viewed by others
References
Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L (2005) Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol 23(1):108–116
Andrasfalvy BK, Mody I (2006) Differences between the scaling of miniature IPSCs and EPSCs recorded in the dendrites of CA1 mouse pyramidal neurons. J Physiol 576(1):191–196
Ascoli GA, Alonso-Nanclares L, Anderson SA et al (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9(7):557–568
Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8(1):45–56
Bayraktar T, Staiger JF, Acsady L, Cozzari C, Freund TF, Zilles K (1997) Co-localization of vasoactive intestinal polypeptide, gamma-aminobutyric acid and choline acetyltransferase in neocortical interneurons of the adult rat. Brain Res 757(2):209–217
Blatow M, Rozov A, Katona I et al (2003) A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38(5):805–817
Burkhalter A (2008) Many specialists for suppressing cortical excitation. Front Neurosci 2(2):155–167
Cardin JA, Carlen M, Meletis K et al (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459(7247):663–667
Caroni P (1997) Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods 71(1):3–9
Cauli B, Audinat E, Lambolez B et al (1997) Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17(10):3894–3906
Cauli B, Porter JT, Tsuzuki K et al (2000) Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA 97(11):6144–6149
Cauli B, Zhou X, Tricoire L, Toussay X, Staiger JF (2014) Revisiting enigmatic cortical calretinin-expressing interneurons. Front Neuroanat 8:52
Chaudhry FA, Reimer RJ, Bellocchio EE et al (1998) The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18(23):9733–9750
Connor JR, Peters A (1984) Vasoactive intestinal polypeptide-immunoreactive neurons in rat visual cortex. Neuroscience 12(4):1027–1044
Cots D, Bosch A, Chillón M (2013) Helper dependent adenovirus vectors: progress and future prospects. Curr Gene Ther 13(5):370–381
Cronin J, Zhang XY, Reiser J (2005) Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5(4):387–398
Defelipe J (1997) Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28 K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14(1):1–19
Demeulemeester H, Vandesande F, Orban GA, Brandon C, Vanderhaeghen JJ (1988) Heterogeneity of GABAergic cells in cat visual cortex. J Neurosci 8(3):988–1000
Dittgen T, Nimmerjahn A, Komai S et al (2004) Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci USA 101(52):18206–18211
Dryga SA, Dryga OA, Schlesinger S (1997) Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology 228(1):74–83
Fechner H, Haack A, Wang H et al (1999) Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther 6(9):1520–1535
Feng G, Mellor RH, Bernstein M et al (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28(1):41–51
Forss-Petter S, Danielson PE, Catsicas S et al (1990) Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron 5(2):187–197
Fremeau RT Jr, Troyer MD, Pahner I et al (2001) The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31(2):247–260
Freund TF, Katona I (2007) Perisomatic inhibition. Neuron 56(1):33–42
Fritschy JM, Harvey RJ, Schwarz G (2008) Gephyrin: where do we stand, where do we go? Trends Neurosci 31(5):257–264
Fujiyama F, Furuta T, Kaneko T (2001) Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol 435(3):379–387
Fujiyama F, Hioki H, Tomioka R et al (2003) Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. J Comp Neurol 465(2):234–249
Fukuda T, Kosaka T (2003) Ultrastructural study of gap junctions between dendrites of parvalbumin-containing GABAergic neurons in various neocortical areas of the adult rat. Neuroscience 120(1):5–20
Fukuda T, Kosaka T, Singer W, Galuske RA (2006) Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network. J Neurosci 26(13):3434–3443
Furuta T, Tomioka R, Taki K, Nakamura K, Tamamaki N, Kaneko T (2001) In vivo transduction of central neurons using recombinant Sindbis virus: golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J Histochem Cytochem 49(12):1497–1508
Furuta T, Timofeeva E, Nakamura K et al (2008) Inhibitory gating of vibrissal inputs in the brainstem. J Neurosci 28(8):1789–1797
Galarreta M, Hestrin S (1999) A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402(6757):72–75
Galarreta M, Hestrin S (2001) Spike transmission and synchrony detection in networks of GABAergic interneurons. Science 292(5525):2295–2299
Galarreta M, Hestrin S (2002) Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA 99(19):12438–12443
Gascon S, Paez-Gomez JA, Diaz-Guerra M, Scheiffele P, Scholl FG (2008) Dual-promoter lentiviral vectors for constitutive and regulated gene expression in neurons. J Neurosci Methods 168(1):104–112
Gibson JR, Beierlein M, Connors BW (1999) Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402(6757):75–79
Gloster A, Wu W, Speelman A et al (1994) The T alpha 1 alpha-tubulin promoter specifies gene expression as a function of neuronal growth and regeneration in transgenic mice. J Neurosci 14(12):7319–7330
Gonchar Y, Burkhalter A (1997) Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7(4):347–358
Gonchar Y, Wang Q, Burkhalter A (2007) Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat 1:3
Gupta A, Wang Y, Markram H (2000) Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science 287(5451):273–278
Herzog E, Bellenchi GC, Gras C et al (2001) The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci 21(22):RC181
Hestrin S, Galarreta M (2005) Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci 28(6):304–309
Hioki H, Fujiyama F, Taki K et al (2003) Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience 117(1):1–6
Hioki H, Fujiyama F, Nakamura K, Wu SX, Matsuda W, Kaneko T (2004) Chemically specific circuit composed of vesicular glutamate transporter 3- and preprotachykinin B-producing interneurons in the rat neocortex. Cereb Cortex 14(11):1266–1275
Hioki H, Kameda H, Nakamura H et al (2007) Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther 14(11):872–882
Hioki H, Kuramoto E, Konno M et al (2009) High-level transgene expression in neurons by lentivirus with Tet-Off system. Neurosci Res 63(2):149–154
Hioki H, Nakamura H, Ma YF et al (2010) Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol 518(5):668–686
Hioki H, Okamoto S, Konno M et al (2013) Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J Neurosci 33(2):544–555
Hu H, Gan J, Jonas P (2014) Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345(6196):1255263
Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T (2009) Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci 12(12):1586–1593
Ito T, Hioki H, Nakamura K et al (2007) Gamma-aminobutyric acid-containing sympathetic preganglionic neurons in rat thoracic spinal cord send their axons to the superior cervical ganglion. J Comp Neurol 502(1):113–125
Jinno S, Kosaka T (2004) Parvalbumin is expressed in glutamatergic and GABAergic corticostriatal pathway in mice. J Comp Neurol 477(2):188–201
Johnson JK, Casagrande VA (1995) Distribution of calcium-binding proteins within the parallel visual pathways of a primate (Galago crassicaudatus). J Comp Neurol 356(2):238–260
Kameda H, Furuta T, Matsuda W et al (2008) Targeting green fluorescent protein to dendritic membrane in central neurons. Neurosci Res 61(1):79–91
Kameda H, Hioki H, Tanaka YH et al (2012) Parvalbumin-producing cortical interneurons receive inhibitory inputs on proximal portions and cortical excitatory inputs on distal dendrites. Eur J Neurosci 35(6):838–854
Kaneko T, Fujiyama F (2002) Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res 42(4):243–250
Kaneko T, Murashima M, Lee T, Mizuno N (1998) Characterization of neocortical non-pyramidal neurons expressing preprotachykinins A and B: a double immunofluorescence study in the rat. Neuroscience 86(3):765–781
Kaneko T, Fujiyama F, Hioki H (2002) Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol 444(1):39–62
Karube F, Kubota Y, Kawaguchi Y (2004) Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J Neurosci 24(12):2853–2865
Kawaguchi Y (1995) Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci 15(4):2638–2655
Kawaguchi Y, Kondo S (2002) Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 31(3–5):277–287
Kawaguchi Y, Kubota Y (1993) Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindin D28 k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70(1):387–396
Kawaguchi Y, Kubota Y (1996) Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci 16(8):2701–2715
Kawaguchi Y, Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7(6):476–486
Kawaguchi Y, Kubota Y (1998) Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience 85(3):677–701
Kinoshita M, Matsui R, Kato S et al (2012) Genetic dissection of the circuit for hand dexterity in primates. Nature 487(7406):235–238
Kubota Y (2014) Untangling GABAergic wiring in the cortical microcircuit. Curr Opin Neurobiol 26:7–14
Kubota Y, Kawaguchi Y (1997) Two distinct subgroups of cholecystokinin-immunoreactive cortical interneurons. Brain Res 752(1–2):175–183
Kubota Y, Hattori R, Yui Y (1994) Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649(1–2):159–173
Kubota Y, Shigematsu N, Karube F et al (2011) Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex 21(8):1803–1817
Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T (2009) Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb Cortex 19(9):2065–2077
Kuramoto E, Ohno S, Furuta T et al (2013) Ventral medial nucleus neurons send thalamocortical afferents more widely and more preferentially to layer 1 than neurons of the ventral anterior-ventral lateral nuclear complex in the rat. Cereb Cortex. doi:10.1093/cercor/bht216
Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B (2013) A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16(11):1662–1670
Letzkus JJ, Wolff SB, Meyer EM et al (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480(7377):331–335
Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6(4):312–324
Lewis DA, Curley AA, Glausier JR, Volk DW (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35(1):57–67
Li L, Suzuki T, Mori N, Greengard P (1993) Identification of a functional silencer element involved in neuron-specific expression of the synapsin I gene. Proc Natl Acad Sci USA 90(4):1460–1464
Ma Y, Hioki H, Konno M et al (2011) Expression of gap junction protein connexin36 in multiple subtypes of GABAergic neurons in adult rat somatosensory cortex. Cereb Cortex 21(11):2639–2649
Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C (2004) Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5(10):793–807
Matsuda W, Furuta T, Nakamura KC et al (2009) Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci 29(2):444–453
Matsuzaki Y, Oue M, Hirai H (2014) Generation of a neurodegenerative disease mouse model using lentiviral vectors carrying an enhanced synapsin I promoter. J Neurosci Methods 223:133–143
Mayford M, Baranes D, Podsypanina K, Kandel ER (1996) The 3′-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci USA 93(23):13250–13255
Meyer AH, Katona I, Blatow M, Rozov A, Monyer H (2002) In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci 22(16):7055–7064
Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM (1998) Development of a self-inactivating lentivirus vector. J Virol 72(10):8150–8157
Mizunuma M, Norimoto H, Tao K et al (2014) Unbalanced excitability underlies offline reactivation of behaviorally activated neurons. Nat Neurosci 17(4):503–505
Moriyama H, Moriyama M, Sawaragi K et al (2013) Tightly regulated and homogeneous transgene expression in human adipose-derived mesenchymal stem cells by lentivirus with tet-off system. PLoS One 8(6):e66274
Nakamura K, Matsumura K, Hübschle T et al (2004) Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24(23):5370–5380
Naldini L, Blomer U, Gallay P et al (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272(5259):263–267
Nishino E, Yamada R, Kuba H et al (2008) Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci 28(28):7153–7164
Ohno S, Kuramoto E, Furuta T et al (2012) A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: a single neuron tracing study with viral vectors. Cereb Cortex 22(12):2840–2857
Oliva AA Jr, Jiang M, Lam T, Smith KL, Swann JW (2000) Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20(9):3354–3368
Orekhova EV, Stroganova TA, Nygren G et al (2007) Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry 62(9):1022–1029
Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M (2013) Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16(8):1068–1076
Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503(7477):521–524
Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS (2008) Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol 100(4):2348–2360
Preuss TM, Kaas JH (1996) Parvalbumin-like immunoreactivity of layer V pyramidal cells in the motor and somatosensory cortex of adult primates. Brain Res 712(2):353–357
Rudy B, McBain CJ (2001) Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24(9):517–526
Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71(1):45–61
Sasahara M, Fries JW, Raines EW et al (1991) PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell 64(1):217–227
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459(7247):698–702
Sohn J, Hioki H, Okamoto S, Kaneko T (2014) Preprodynorphin-expressing neurons constitute a large subgroup of somatostatin-expressing GABAergic interneurons in the mouse neocortex. J Comp Neurol 522(7):1506–1526
Somogyi P (1977) A specific ‘axo-axonal’ interneuron in the visual cortex of the rat. Brain Res 136(2):345–350
Somogyi P, Klausberger T (2005) Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562(1):9–26
Somogyi P, Tamas G, Lujan R, Buhl EH (1998) Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26(2–3):113–135
Spatz WB, Illing RB, Weisenhorn DM (1994) Distribution of cytochrome oxidase and parvalbumin in the primary visual cortex of the adult and neonate monkey, Callithrix jacchus. J Comp Neurol 339(4):519–534
Stichel CC, Singer W, Heizmann CW, Norman AW (1987) Immunohistochemical localization of calcium-binding proteins, parvalbumin and calbindin-D28 k, in the adult and developing visual cortex of cats: a light and electron microscopic study. J Comp Neurol 262(4):563–577
Taki K, Kaneko T, Mizuno N (2000) A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience 98(2):221–231
Tamamaki N, Nakamura K, Furuta T, Asamoto K, Kaneko T (2000) Neurons in Golgi-stain-like images revealed by GFP-adenovirus infection in vivo. Neurosci Res 38(3):231–236
Tamas G, Buhl EH, Somogyi P (1997) Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol 500(3):715–738
Tamas G, Somogyi P, Buhl EH (1998) Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci 18(11):4255–4270
Tamas G, Buhl EH, Lorincz A, Somogyi P (2000) Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci 3(4):366–371
Tanahira C, Higo S, Watanabe K et al (2009) Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neurosci Res 63(3):213–223
Tanaka YH, Tanaka YR, Fujiyama F, Furuta T, Yanagawa Y, Kaneko T (2011a) Local connections of layer 5 GABAergic interneurons to corticospinal neurons. Front Neural Circuits 5:12
Tanaka YR, Tanaka YH, Konno M et al (2011b) Local connections of excitatory neurons to corticothalamic neurons in the rat barrel cortex. J Neurosci 31(50):18223–18236
Thomson AM, Lamy C (2007) Functional maps of neocortical local circuitry. Front Neurosci 1(1):19–42
Thomson AM, West DC, Hahn J, Deuchars J (1996) Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol 496(1):81–102
Tomioka R, Rockland KS (2006) Improved Golgi-like visualization in retrogradely projecting neurons after EGFP-adenovirus infection in adult rat and monkey. J Histochem Cytochem 54(5):539–548
Tomioka R, Rockland KS (2007) Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol 505(5):526–538
Tomko RP, Johansson CB, Totrov M, Abagyan R, Frisén J, Philipson L (2000) Expression of the adenovirus receptor and its interaction with the fiber knob. Exp Cell Res 255(1):47–55
Toribio R, Ventoso I (2010) Inhibition of host translation by virus infection in vivo. Proc Natl Acad Sci USA 107(21):9837–9842
Uematsu M, Hirai Y, Karube F et al (2008) Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex 18(2):315–330
van Brederode JF, Helliesen MK, Hendrickson AE (1991) Distribution of the calcium-binding proteins parvalbumin and calbindin-D28 k in the sensorimotor cortex of the rat. Neuroscience 44(1):157–171
Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD (2002) Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 22(1):142–155
Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H (2002) Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex 12(4):395–410
Watakabe A, Kato S, Kobayashi K et al (2012) Visualization of cortical projection neurons with retrograde TET-off lentiviral vector. PLoS One 7(10):e46157
Whittington MA, Traub RD, Jefferys JG (1995) Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373(6515):612–615
Williams SR, Stuart GJ (2003) Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci 23(19):7358–7367
Woodruff A, Xu Q, Anderson SA, Yuste R (2009) Depolarizing effect of neocortical chandelier neurons. Frontiers in neural circuits 3:15
Wouterlood FG, Hartig W, Bruckner G, Witter MP (1995) Parvalbuminimmunoreactive neurons in the entorhinal cortex of the rat: localization, morphology, connectivity and ultrastructure. J Neurocytol 24(2):135–153
Xu X, Roby KD, Callaway EM (2006) Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol 499(1):144–160
Xu X, Roby KD, Callaway EM (2010) Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518(3):389–404
Yin DX, Zhu L, Schimke RT (1996) Tetracycline-controlled gene expression system achieves high-level and quantitative control of gene expression. Anal Biochem 235(2):195–201
Zaiss AK, Son S, Chang LJ (2002) RNA 3′ readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J Virol 76(14):7209–7219
Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kröner S, Lewis DA, Krimer LS (2005) Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15(8):1178–1186
Zaitsev AV, Povysheva NV, Gonzalez-Burgos G et al (2009) Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex 19(7):1597–1615
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 15(9):871–875
Acknowledgments
The author greatly thanks Prof. Takeshi Kaneko and the lab members at the Department of Morphological Brain Science, Graduate School of Medicine, Kyoto University for their valuable discussions and collaborations. This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas “Neural Diversity and Neocortical Organization” (25123709) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) and a Grant-in-Aid for Scientific Research (C) (24500408) from the Japan Society for the Promotion of Science (JSPS). The author received the Incitement Award of the Japanese Association of Anatomists in Japan for the fiscal year 2013, and gave a presentation of the present review at the 119th Annual Meeting in Shimono, Japan on March 28, 2014.
Conflict of interest
The author has no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hioki, H. Compartmental organization of synaptic inputs to parvalbumin-expressing GABAergic neurons in mouse primary somatosensory cortex. Anat Sci Int 90, 7–21 (2015). https://doi.org/10.1007/s12565-014-0264-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-014-0264-8