Abstract
The purpose of this study was to quantify the transfer of viral and bacterial pathogens in water used to dilute pesticides sprayed onto the surfaces of cantaloupe, iceberg lettuce, and bell peppers. The average percent transfer of bacteria was estimated to range from 0.00021 to 9.4%, while average viral transfer ranged from 0.055 to 4.2%, depending on the type of produce. Based on these values the concentrations of hepatitis A virus (HAV) and Salmonella in water necessary to achieve a 1:10,000 annual risk of infection were calculated. Under worst case scenario assumptions, in which a pesticide is applied on the same day that the produce is harvested and when maximum transfer values are used, concentrations of 1.5 × 10−3 CFU Salmonella or 2.7 × 10−7 MPN HAV per 100 ml of the water used for application would result in 1:10,000 annual infection risk to anyone who consumes the fresh produce. If harvesting does not occur until at least 14 days after the application, to produce the same risk of infection, the numbers of Salmonella in 100 ml of water used to dilute the pesticides will be greater by up to five orders of magnitude, while the HAV numbers will have increased by up to two orders of magnitude. Based on the reported concentrations of enteric viruses in surface and ground waters in the United States, a 1:10,000 annual risk of infection could easily be exceeded with some groundwater sources used in the United States. To reduce the risks associated with the consumption of fresh produce, water used to prepare pesticides in spray applications should be evaluated for its microbiological quality.
Similar content being viewed by others
Introduction
Over the past few decades the number of foodborne diseases associated with the consumption of fresh produce has increased in the United States (Lynch et al. 2009). Many types of fresh fruits and vegetables are consumed raw or minimally prepared, and most have fewer barriers against microbial growth, such as salt and preservatives, than do the more traditional vehicles of foodborne illness, such as undercooked meat and poultry (Tauxe 1997). Due to this lack, and also because of the survival and growth of microbial pathogens on such foods, fresh produce is expected to continue to cause outbreaks of foodborne illnesses (Meng and Doyle 2002). Foods such as tomatoes, cantaloupe, green onions, lettuce, and frozen raspberries especially have been associated with foodborne illness caused by such enteric pathogens as hepatitis A virus (HAV) (CDC 2003; Reid and Robinson 1987; Rosenblum et al. 1990) and Salmonella (Hedberg et al. 1994). Sources of such contaminants can be feces, soil, human hands, and irrigation water (Beuchat 1995). Irrigation water in turn can become contaminated by a variety of vectors, including wild and domestic animals, flood waters, wastewaters, recreational users (swimmers), and runoff from livestock production operations (Beuchat 1995; FDA 1998). Irrigation water contaminated by any one or more of these vectors can subsequently contaminate crops and soil it is applied to, and eventually the pathogens can pose a health risk to consumers as well as risk to the field workers (Pianietti et al. 2004).
Water routinely used to dilute pesticides can also become contaminated and subsequently cause illness in a similar fashion. In 1996, for example, an outbreak of cyclosporiasis, associated with contaminated Guatemalan raspberries, occurred in North America (Herwaldt et al. 1997), and after a subsequent outbreak in 1997, it was determined that the raspberries may have been contaminated by fungicides and insecticides diluted with water that had been contaminated by human feces (Caceres et al. 1998; Herwaldt and Beach 1999). Sathyanarayanan and Ortega (2004) found that various pesticides do not affect the sporulation of Cyclospora when used at recommended concentration levels, thus further indicating the danger of mixing contaminated water with pesticides applied by spraying.
The microbial standards for irrigation water have primarily been evaluated with respect to the use of reclaimed wastewater as an irrigation source (Asano et al. 1992; Petterson et al. 2001; Shuval et al. 1997; WHO 1989). However, a previous evaluation of the quality of surface water used for irrigation has led to a suggested bacteriological standard of 1,000 fecal coliforms per 100 ml (Geldreich and Bordner 1971). Currently, there is no consensus on the acceptable level of any risk of infection associated with the consumption of food. However, the U. S. Environmental Protection Agency’s (USEPA) benchmark for the acceptable annual risk of infection due to the consumption of drinking water is 1:10,000 (Regli et al. 1991), and this value has been used to assess the risk associated with the consumption of fresh produce (Manshadi 2003; Tanaka et al. 1998). The primary goal of this study was to evaluate the transfer of microorganisms from contaminated water to the surfaces of fresh produce via pesticide spray application, and estimate the risk of infection for hepatitis A and Salmonella from ingestion of the contaminated produce.
Materials and Methods
Preparation and Assay of Microorganisms
Coliphage PRD1 was selected as a model for hepatitis A virus as we have previously shown it had a similar survival on produce (Stine et al. 2005a). The same was true for the selection of E. coli as a model for Salmonella (Stine et al. 2005a). PRD1 coliphage was obtained from the University of Arizona Department of Soil, Water, and Environmental Science and propagated using Salmonella typhimurium ATCC 19585 (Governal and Gerba 1997). Escherichia coli ATCC 25922 (American Type Culture Collection, ATCC, Rockville, MD) was grown for 18–24 h in tryptic soy broth (Difco Company, Detroit, MI) at 37°C. E. coli ATCC 25922 was assayed using the Colilert quanti-tray system (IDEXX, Westbrook, MA); while PRD1 was assayed in duplicate using the plaque-forming unit method (Governal and Gerba 1997).
Microbial Inoculation and Sampling
Experiments were conducted at the University of Arizona Campus Agricultural Center in Tucson, AZ, USA. Three separate plots were used to grow cantaloupe (Mission variety hybrid, Willhite Seed Inc., TX, USA), iceberg lettuce (Beacon variety, Paragon Seed Inc., CA, USA), and bell peppers (California Wonder, Willhite Seed Inc., Poolville, TX, USA) in sandy loam soil. The plots, each measuring 10 m × 4 m, were oriented in a north–south direction. Starting and ending dates of each experiment are reported in Table 1. Groundwater was used to irrigate crops. The plots were irrigated using a subsurface irrigation system (SDI). SDI systems introduced irrigation water directly to the roots of the plant because this type of system offers advantages over other irrigation methods in terms of crop yield as well as water use efficiency. For example, in situations that involved using reclaimed wastewater to irrigate, SDI minimizes the chances that agricultural products and/or field workers to be exposed to the irrigation water and, consequently, be contaminated by microbial pathogens (Choi et al. 2004).
A sprayer (Flomaster, 9.5 l capacity, Root-Lowell Manufacturing Co., Lowell, MI) was filled with 9 l of irrigation water, and then PRD1 coliphage and E. coli ATCC 25922 were added to the water to a concentration of approximately 105/ml each. The contents of the spray canister were mixed and administered to the SDI plot during the early morning to minimize the influence of the wind. Before each experiment, an N,N-diethyl-p-phenylene diamine (DPD) colorimetric test (Hach Chemical Co., Loveland, CO, USA) was used to determine that the irrigation water contained no free chlorine. For each experiment, three samples of the edible portions of each plant were collected from each irrigation plot on days 0, 1, 3, 5, 7, 10, and 14. To obtain lettuce samples, each lettuce head was cut from the stem and, in keeping with industry harvesting methods, the two or three outermost leaves were removed. Cantaloupe melons were cut from the vine, and soil was subsequently brushed off each melon. To obtain samples of bell peppers, the fruit was removed from the stem by hand. The coliphage and E. coli were qualified on the produce surface by the method described by Stine et al. (2005a). The environmental conditions under which each experiment was conducted are shown in Table 1.
Inactivation Rate
In recent investigation of pre-harvest survival of enteric pathogens on the surfaces of fresh produce (Stine et al. 2005a), the inactivation rates of hepatitis A virus (HAV) and Salmonella on the surfaces of cantaloupe, lettuce, and bell pepper were estimated to be 0.01, 0.12, and 0.11 day−1 and 0.24, 0.35, and 0.20 day−1, respectively. It was assumed that the microbial rates of inactivation were not affected by the presence of pesticides (Pham et al. 2004).
Concentration of Microorganisms in Water Necessary to Achieve 1:10,000 Yearly Risk
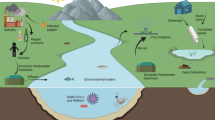
Figure 1 provides an overview of the steps taken and the assumptions made in the calculations to determine the concentrations of microorganisms in the water that we had mixed with the pesticide spray to achieve the specified 1:10,000 annual risk of infection. The equation
was used to calculate daily acceptable risk (P i), where P A is the annual risk (Haas et al. 1999). The annual acceptable risk was assumed to be 1:10,000. The beta-Poisson dose–response model (Haas et al. 1999) was used to determine the dose (d) of a microorganism necessary to achieve the calculated P i value
where N 50 and α are the median infectious dose and the parameter defining the dose–response curve, respectively. The dose (d) of a microorganism needed to achieve the necessary P i value was also calculated using the exponential dose–response model (Mena et al. 2003)
where k is an exponential parameter. The dose–response parameter k for hepatitis A virus was assumed to be 1.8229. For Salmonella, dose–response parameter values of 2.36 × 104 and 0.3126 were used for N 50, and α, respectively (Haas et al. 1999). To determine the amount of each organism that would have to be present per gram of each fresh produce in order to produce annual acceptable risk, we divided d by the amount of produce items (g) consumed. Based on the assumption that on average per capita in the United States annually consumes 3248.5 g of bell peppers, 4662.8 g of cantaloupes, and 4416.5 g of lettuce (Alum 2001; USDA 2002), we calculated the microbial densities that would have to be present in the irrigation water to achieve the acceptable annual risk. These numbers were obtained by dividing the amount of each produce item consumed in the United States by the fraction of microorganisms transferred to the surface of produce items from the water used in the spray application of pesticides. PRD1 and E. coli ATCC 25922 were used as surrogates to determine the fractions of enteric virus and bacteria that contaminated the produce via pesticide spray application. These fractions were calculated from both the arithmetic mean and the maximum transfer values. Because surfaces of melons are typically not consumed, the risk arises when pathogens are transferred into the edible flesh during slicing. The percentage of microorganisms that were transferred from the irrigation water to contaminate the surface of cantaloupe was multiplied by the fraction of microorganisms present on the melon surface and recovered from the flesh after such transfers. A recent study estimated the percent recoveries to be 0.34% for PRD1 coliphage and 1.4% for E. coli ATCC 25922 (Stine et al. 2005b). The numbers of HAV and Salmonella that would have to be present in water used to prepare pesticide spray for there to be a 1:10,000 annual risk of infection were calculated based on the assumption that the last pesticide spray application event took place either on the same day as the harvest or 14 days before harvest. We also assumed that no post-harvest inactivation of enteric pathogens occurred before the consumption of contaminated fresh produce, as minimal inactivation of pathogens has been described in post-harvest conditions (Behrsing et al. 2003; Croci et al. 2002).
Results
Transfer of Microorganisms from Water to Produce Surface
The fractions of microorganisms transferred to the surfaces of cantaloupe, iceberg lettuce, and bell peppers from water used for pesticide spray application are reported in Table 2. The largest fractions of PRD1 and E. coli transferred to the surfaces of produce occurred on lettuce and bell peppers, respectively.
Microbial Concentrations in Water to Achieve 1:10,000 Risk of Infection
When harvesting occurred on the same day as spraying, the numbers of HAV that were needed in the pesticide water to present a risk of infection of 1:10,000 were as low as 2.7 × 10−7 most probable number (MPN)/100 ml (Table 3). For Salmonella the corresponding numbers were as low as 1.5 × 10−3 CFU/100 ml (Table 4). When harvesting does not occur until 14 days after the last pesticide spray application, the numbers of Salmonella in the water used to dilute the pesticides would have to increase by up to five orders of magnitude, while HAV numbers would have to increase by up to two orders of magnitude before consumers would be exposed to the 1:10,000 annual risk of infection.
Discussion
Enteroviruses have been found to occur in surface waters at concentrations of 6.7 × 10−2 to 0.544 MPN MPN/100 ml (Lucena et al. 1985) and more recently at concentrations of 1.7 × 10−4 to 5.9 × 10−4 infectious units (IU)/100 ml, in watersheds affected by onsite sewage disposal systems (Lipp et al. 2001). Based on these data, the annual risk of infection would appear to be equal to, or to exceed, a 1:10,000 annual risk if the surface waters in the cited studies were used in pesticide spray application on lettuce and bell pepper crops. The concentrations of HAV that would be needed to achieve a 1:10,000 risk of infection from cantaloupe are also less than or within the range of concentrations of HAV, enterovirus, rotavirus, and norovirus, which were estimated to be 9 × 10−5 to 1.86 × 10−3 MPN/100 ml in groundwater samples taken from sources in the United States and which tested positive for viruses (Abbaszadengan et al. 2003). The concentrations of enteric viruses in disinfected secondary effluent have been reported to range from 3.5 × 10−3 to 0.65 PFU/100 ml in the United States (NRC 1998). Therefore, because of the level of enteric viruses present, applying a pesticide that had been diluted with secondary effluent from a wastewater treatment plant would result in significant risks of infection to anyone consuming cantaloupe, lettuce, or bell peppers that had been sprayed with such a mixture.
Based on calculated Salmonella numbers, under the worst case scenario, wastewaters secondarily treated by primary sedimentation, trickling filter or activated sludge, disinfection, coagulation, filtration, and disinfection could all be used to prepare pesticide spray used on cantaloupe, bell peppers, and lettuce without exceeding the 1:10,000 annual risk of infection to consumers, assuming a Salmonella concentration of 4.0 × 10−5 to 70 CFU/100 ml (Maier et al. 2009). However, the contamination of soil and the production of bioaerosols resulting from the spraying pesticides diluted with contaminated water could open other routes of produce contamination, as well as the infection of field workers (Pianietti et al. 2004).
While it was assumed that the enteric pathogens evaluated in this study were not affected by the presence of pesticides, Pham et al. (2004) have reported some inactivation of E. coli by pesticides. The influence of various fungicides and insecticides on the sporulation of Cyclospora cayetanensis and infectivity of Cryptosporidium parvum has been evaluated (Sathyanarayanan and Ortega 2004). C. parvum infectivity was found to be reduced after 1 week of incubation at pesticide treatment concentrations higher than the recommended dosage. The impact of various pesticide spray formulations on viruses has not previously been reported. However, human enteric viruses are very stable under a wide range of pH and water quality conditions (Maier et al. 2009). Further studies are needed to gain a better understanding of the conditions under which survival of enteric pathogens survive and multiply in water to dilute pesticide sprays, and also the microbial risks associated with those sprays.
References
Abbaszadengan, M., LeChevallier, M., & Gerba, C. P. (2003). Occurrence of viruses in US groundwater. Journal of the American Water Works Association, 95(9), 107–120.
Alum, A. (2001). Control of viral contamination of reclaimed irrigated vegetables by drip irrigation. Doctoral dissertation. University of Arizona, Tucson, AZ.
Asano, T., Leong, L. Y. C., Rigby, M. G., & Sakaji, R. H. (1992). Evaluation of the California wastewater reclamation criteria using enteric virus monitoring data. Water Science and Technology, 26, 1513–1524.
Behrsing, J., Jaeger, J., Horlock, F., Kita, N., Franz, P., & Premier, R. (2003). Survival of Listeria innocua, Salmonella salford and Escherichia coli on the surface of fruit with inedible skin. Postharvest Biology and Technology, 29, 249–256.
Beuchat, L. R. (1995). Pathogenic microorganisms associated with fresh produce. Journal of Food Protection, 59, 204–216.
Caceres, V. M., Ball, R. T., Somerfeldt, S. A., Mackey, R. L., Nichols, S. E., MacKenzie, W. R., et al. (1998). A foodborne outbreak of cyclosporiasis caused by imported raspberries. Journal of Family Practice, 47, 231–234.
Center for Disease Control, Prevention (CDC). (2003). Hepatitis A outbreak associated with green onions at a restaurant-Monaca, Pennsylvania, 2003. Morbidity and Mortality Weekly Reports, 52, 1155–1157.
Choi, C., Song, I., Stine, S., Pimentel, J., & Gerba, C. (2004). Role of irrigation and wastewater reuse: comparison of subsurface irrigation and furrow irrigation. Water Science and Technology, 50, 61–68.
Croci, L., De Medici, D., Scalfaro, C., Fiore, A., & Toti, L. (2002). The survival of hepatitis A virus in fresh produce. International Journal of Food Microbiology, 73, 29–34.
Drug Food Administration (FDA). (1998). Guide to minimize microbial food safety hazards for fresh fruits and vegetables. Washington, DC: Center for Food Safety and Applied Nutrition.
Geldreich, E. E., & Bordner, R. H. (1971). Fecal contamination of fruits and vegetables during cultivation and processing for market. A review. Journal Milk and Food Technology, 34, 184–195.
Governal, R. A., & Gerba, C. P. (1997). Persistence of MS-2 and PRD1 bacteriophages in an ultra-pure water system. Journal of Industrial Microbiology & Biotechnology, 18, 297–301.
Haas, C. N., Rose, J. B., & Gerba, C. P. (1999). Quantitative microbial risk assessment. New York: Wiley.
Hedberg, C. W., MacDonald, K. L., & Osterholm, M. T. (1994). Changing epidemiology of food-borne disease: a Minnesota perspective. Clinical Infectious Diseases, 18, 671–682.
Herwaldt, B. L., Acker, M. L., Farrar, J., Richardson, S., Nelson, R., Fletcher, M., et al. (1997). An outbreak in 1996 of cyclosporiasis associated with imported raspberries. New England Journal of Medicine, 336, 1548–1556.
Herwaldt, B. L., & Beach, M. J. (1999). The return of Cyclospora in 1997: Another outbreak of cyclosporiasis in North America associated with imported raspberries. Annuals of Internal Medicine, 130, 210–220.
Lipp, E. K., Farrah, S. A., & Rose, J. B. (2001). Assessment and impact of microbial fecal pollution and human enteric pathogens in a coastal community. Marine Pollution Bulletin, 42, 286–293.
Lucena, F., Bosch, A., Jofre, J., & Schwartzbrod, L. (1985). Identification of viruses isolated from sewage, river water, and coastal seawater in Barcelona. Water Research, 19, 1237–1239.
Lynch, M. F., Tauxe, R. V., & Hedberg, C. W. (2009). The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiology and Infection, 137, 307–315.
Maier, R. M., Pepper, I. L., & Gerba, C. P. (2009). Environmental microbiology (2nd ed.). New York: Academic Press.
Manshadi, F. D. (2003). Occurrence of pathogenic and indicator microorganisms on produce irrigated with dairy wastewater. Doctoral Dissertation. University of Arizona, Tucson, AZ.
Mena, K. D., Gerba, C. P., Haas, C. N., & Rose, J. B. (2003). Risk assessment of waterborne coxsackievirus. Journal of the American Water Works Association, 95(7), 122–131.
Meng, J., & Doyle, M. P. (2002). Introduction. Microbial food safety. Microbes and Infection, 4, 395–397.
National Research Council (NRC). (1998). Issues in potable reuse. Washington, DC: National Academy Press.
Petterson, S. R., Ashbolt, N. J., & Sharma, A. (2001). Microbial risks from wastewater irrigation of salad crops: A screening-level risk assessment. Water Environment Research, 72, 667–672.
Pham, C. H., Min, J., & Gu, B. (2004). Pesticide induced toxicity and stress response in bacterial cells. Bulletin of Environment Contamination and Toxicology, 72, 380–386.
Pianietti, A., Sabatini, L., Bruscolini, F., Chiaverini, F., & Cecchetti, G. (2004). Fecal contamination indicators, Salmonella, Vibrio and Aeromonas in water used for the irrigation of agricultural products. Epidemiology and Infection, 132, 231–238.
Regli, S., Rose, J. B., Haas, C. N., & Gerba, C. P. (1991). Modeling the risk from Giardia and viruses in drinking water. Journal of the American Water Works Association, 83(11), 76–84.
Reid, T. M. S., & Robinson, H. G. (1987). Frozen raspberries and hepatitis A. Epidemiology and Infection, 98, 109–112.
Rosenblum, L. S., Mirkin, I. R., Allen, D. T., Safford, S., & Hadler, S. C. (1990). A multifocal outbreak of hepatitis A traced to commercially distributed lettuce. American Journal of Public Health, 80, 1075–1079.
Sathyanarayanan, L., & Ortega, Y. (2004). Effects of pesticides on sporulation of Cyclospora cayetanensis and viability of Cryptosporidium parvum. Journal of Food Protection, 67, 1044–1049.
Shuval, H., Lampert, Y., & Fattal, B. (1997). Development of a risk assessment approach for evaluating wastewater reuse standards for agriculture. Water Science and Technology, 35, 15–20.
Stine, S. W., Song, I., Choi, C. Y., & Gerba, C. P. (2005a). Effect of relative humidity on preharvest survival of bacterial and viral pathogens on the surface of cantaloupe, lettuce, and bell peppers. Journal of Food Protection, 68, 1352–1358.
Stine, S. W., Song, I., Choi, C. Y., & Gerba, C. P. (2005b). Application of microbial risk assessment to the development of standards for enteric pathogens in irrigation water. Journal of Food Protection, 68, 913–918.
Tanaka, H., Asano, T., Schroeder, E. D., & Tchobanoglous, G. (1998). Estimating the safety of wastewater reclamation and reuse using enteric virus monitoring data. Water Environment Research, 70, 39–51.
Tauxe, R. V. (1997). Emerging foodborne diseases: An evolving public health challenge. Emerging Infectious Diseases, 3, 425–434.
United States Department of Agriculture (USDA). (2002). Food consumption (per capita) data. http://ers.usda.gov/data/foodconsumption/datasystem.asp. Accessed April 16, 2008.
World Health Organization (WHO). (1989). Health guidelines for the use of wastewater in agriculture and aquaculture. Report of a WHO scientific group, Geneva, WHO.
Acknowledgment
This work was supported by the CFSAN grant from the U. S. Food and Drug Administration (Grant No. FD-U-002109-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stine, S.W., Song, I., Choi, C.Y. et al. Application of Pesticide Sprays to Fresh Produce: A Risk Assessment for Hepatitis A and Salmonella . Food Environ Virol 3, 86–91 (2011). https://doi.org/10.1007/s12560-011-9061-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-011-9061-x