Abstract
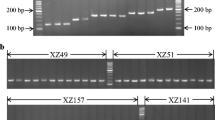
Cordyceps militaris, the type species of genus Cordyceps, is one of the most popular mushrooms and a nutraceutical in eastern Asia. It is considered a model organism for the study of Cordyceps species because it can complete its life cycle when cultured in vitro. In the present study, the occurrence and sequence variation of SSU rDNA group I introns, Cmi.S943 and Cmi.S1199, among different isolates of C. militaris were analyzed. Based on the secondary structure predictions, the Cmi.S943 intron has been placed in subgroup IC1, and the Cmi.S1199 intron has been placed in subgroup IE. No significant similarity between Cmi.S943 and Cmi.S1199 suggested different origins. Three genotypes, based on the frequency and distribution of introns, were described to discriminate the 57 surveyed C. militaris strains. It was found that the genotype was related to the stroma characteristics. The stromata of all of the genotype II strains, which possessed only Cmi.S943, could produce perithecium. In contrast, the stromata of all genotype III strains, which had both Cmi.S943 and Cmi.S1199, could not produce perithecium. Cmi.S1199 showed the lowest level of intra-specific variation among the tested strains. Group I introns can be lost during strain cross-mating. Therefore, we presumed that during cross-mating and recombination, intron loss could be driven by positive Darwinian selection due to the energetic cost of transcribing long introns.
Similar content being viewed by others
References
Burke, J.M., Belfort, M., Cech, T.R., Davies, R.W., Schweyen, R.J., Shub, D.A., Szostak, J.W., and Tabak, H.F. 1987. Structural convention for group I introns. Nucleic Acids Res. 15, 7217–7221.
Cech, T.R. 1988. Conserved sequences and structures of group I introns: building an active site for RNA catalysis-a review. Gene 73, 259–271.
Coates, B., Hellmich, R.L., and Lewis, L.C. 2002. Nuclear small subunit rRNA group I intron variation among Beauveria spp. provide tools for strain identification and evidence of horizontal transfer. Curr. Genet. 41, 414–424.
Côté, M.J., Prud’homme, M., Meldrum, A.J., and Tardif, M.C. 2004. Variations in sequence and occurrence of SSU rDNA group I introns in Monilinia fructicola isolates. Mycologia 96, 240–248.
Doyle, J.J. and Doyle, J.L. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15.
Feau, N., Hamelin, R.C., and Bernier, L. 2007. Variability of nuclear SSU-rDNA group introns within Septoria species: incongruence with host sequence phylogenies. J. Mol. Evol. 64, 489–499.
Gargas, A. and Taylor, J.W. 1992. Polymerase chain reaction (PCR) primers for amplifying and sequencing nuclear 18S rDNA from lichenized fungi. Mycologia 84, 589–592.
Gutell, R.R. 1993. Collection of small subunit (16S- and 16S-like) ribosomal RNA structures. Nucleic Acids Res. 21, 3051–3054.
Hafez, M., Iranpour, M., Mullineux, S.T., Sethuraman, J., Wosnitza, K.M., Lehn, P., Kroeker, J., Loewen, P.C., Reid, J., and Hausner, G. 2012. Identification of group I introns within the SSU rDNA gene in species of Ceratocystiopsis and related taxa. Fungal Biol. 116, 98–111.
Johansen, S. and Haugen, P. 2001. A new nomenclature of group I introns in ribosomal DNA. RNA 7, 935–936.
Lambowitz, A.M. and Belfort, M. 1993. Introns as mobile genetic elements. Annu. Rev. Biochem. 62, 587–622.
Li, Z.J. and Zhang, Y. 2005. Predicting the secondary structures and tertiary interactions of 211 group I introns in IE subgroup. Nucleic Acids Res. 33, 2118–2128.
Llopart, A., Comeron, J.M., Brunet, F.G., Lachaise, D., and Long, M. 2002. Intron presence-absence polymorphism in Drosophila driven by positive Darwinian selection. Proc. Natl. Acad. Sci. USA 99, 8121–8126.
Machouart, M., Gueidan, C., Khemisti, A., Dulongcourty, R., Sudhadham, M., and de Hoog, G.S. 2011. Use of ribosomal introns as new markers of genetic diversity in Exophiala dermatitidis. Fungal Biol. 115, 1038–1050.
Michel, F. and Westhof, E. 1990. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J. Mol. Biol. 216, 585–610.
Nikoh, N. and Fukatsu, T. 2001. Evolutionary dynamics of multiple group I introns in nuclear ribosomal RNA genes of endoparasitic fungi of the genus Cordyceps. Mol. Biol. Evol. 18, 1631–1642.
Perotto, S., Nepote-Fus, P., Saletta, L., Bandi, C., and Young, J.P.W. 2000. A diverse population of introns in the nuclear ribosomal genes of ericoid mycorrhizal fungi includes elements with sequence similarity to endonuclease-coding genes. Mol. Biol. Evol. 17, 44–59.
Rijk, P.D., Wuyts, J., and Wachter, R.D. 2003. RnaViz 2: an improved representation of RNA secondary structure. Bioinformatics 19, 299–300.
Shinohara, M.L., LoBuglio, K.F., and Rogers, S.O. 1996. Group I intron family in the nuclear ribosomal RNA small subunit genes of Cenococcum geophilum isolates. Curr. Genet. 29, 377–387.
Shrestha, B., Choi, S.K., Kim, H.K., Kim, T.W., and Sung, J.M. 2004. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotechnol. Bioprocess. Eng. 9, 440–446.
Shrestha, B., Zhang, W., Zhang, Y., and Liu, X.Z. 2012. The medicinal fungus Cordyceps militaris: research and development. Mycol. Progress 11, 599–614.
Suh, S.O., Jones, K.G., and Blackwell, M. 1999. A group I intron in the nuclear small subunit rRNA gene of Cryptendoxyla hypophloia, an ascomycetous fungus: evidence for a new major class of group I introns. J. Mol. Evol. 48, 493–500.
Sung, G.H., Hywel-Jones, N.L., Sung, J.M., Luangsa-Ard, J.J., Shrestha, B., and Spatafora, J.W. 2007. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 57, 5–59.
Takizawa, K., Hashizume, T., and Kamei, K. 2011. Occurrence and characteristics of group I introns found at three different positions within the 28S ribosomal RNA gene of the dematiaceous Phialophora verrucosa: phylogenetic and secondary structural implications. BMC Microbiol. 11, 94.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739.
White, T.J., Burns, T., Lee, S., and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315–322. In Innis, M.A., Gelfand, D.H., Sninsky, J.J., and White, T.J. (eds.), PCR Protocols: a guide to methods and applications. Academic Press, New York, USA.
Xu, C., Wang, C., Sun, X., Zhang, R., Gleason, M.L., Eiji, T., and Sun, G. 2013. Multiple group I introns in the small-subunit rDNA of Botryosphaeria dothidea: Implication for intra-specific genetic diversity. PLoS ONE 8, e678–8.
Yokoyama, E., Arakawa, M., Yamagishi, K., and Hara, A. 2006. Phylogenetic and structural analyses of the mating-type loci in Clavicipitaceae. FEMS Microbiol. Lett. 264, 182–191.
Zhan, Y., Dong, C.H., and Yao, Y.J. 2006. Antioxidant activities of aqueous extract from cultivated fruit-bodies of Cordyceps militaris (L.) Link in vitro. J. Integr. Plant Biol. 48, 1365–1370.
Zhang, Y.J., Li, E.W., Wang, C.S., Li, Y.L., and Liu, X.Z. 2012. Ophiocordyceps sinensis, the flagship fungus of China: terminology, life strategy and ecology. Mycology 3, 2–10.
Zhu, T. and Niu, D.K. 2013. Frequency of intron loss correlates with processed pseudogene abundance: a novel strategy to test the reverse transcriptase model of intron loss. BMC Biol. 11, 23.
Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lian, T., Yang, T., Yang, T. et al. Variations of SSU rDNA group I introns in different isolates of Cordyceps militaris and the loss of an intron during cross-mating. J Microbiol. 52, 659–666 (2014). https://doi.org/10.1007/s12275-014-3681-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-014-3681-4