Abstract
Repeated surveys of the Kennebec estuary, a macrotidal river estuary in Maine, USA, between 2004 and 2008 found spatial and temporal variability both in sources of carbon dioxide (CO2) to the estuary and the air–sea flux of estuary CO2. On an annual basis, the surveyed area of the Kennebec estuary had an area-weighted average partial pressure of CO2 (pCO2) of 559 μatm. The area-weighted average CO2 flux to the atmosphere was 3.54 mol C m−2 year−1. Overall, the Kennebec estuary was an annual source of 7.2 × 107 mol CO2 to the atmosphere. Distinct seasonality in estuarine pCO2 was observed, with shifts in the seasonal pattern evident between lower and higher salinities. Fluxes of CO2 from the estuary were elevated following two summertime storms, and inputs of riverine CO2 outweighed internal estuarine CO2 inputs in nearly all months. River and estuarine inputs of CO2 represented 68 and 32 % of the total CO2 contributions to the estuary, respectively. This study examines the variability of CO2 in a large New England estuary, and highlights the comparatively high contribution of CO2 from riverine sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The flux of water through estuaries represents a relatively small portion of the global water budget, and the surface area of estuaries is small relative to the surface area of the coastal ocean over the continental shelves. However, estuaries play a disproportionate role in the transport and transformation of carbon, both organic and inorganic, from land to sea (Borges 2005; Gattuso et al. 1998). Through coupled biological and physical processes, estuaries are generally strong sources of carbon dioxide (CO2) release to the atmosphere (Borges 2005; Cai et al. 2006; Laruelle et al. 2010; Borges and Abril 2011). The partial pressure of CO2 (pCO2) in estuaries, while usually above atmospheric levels, can range over several orders of magnitude. In an extreme example, pCO2 in the Indian Chilka lagoon ranged from 83 to 6,522 μatm (Gupta et al. 2008). Some recent work has suggested that the release of CO2 from estuaries is high enough in magnitude to offset the uptake of CO2 over the continental shelves (Cai 2011).
The typical high rates of estuarine outgassing of excess CO2 are sustained by organic and inorganic carbon from two sources: river and groundwater inputs and inputs from the surrounding estuary and coastal wetlands and marshes. Estuaries receive large loads of allochthonous carbon from rivers, and can modify these inputs as they are mixed through the estuaries and into the coastal ocean (Salisbury et al. 2009). At the same time, estuaries can also provide large inputs of autochthonous carbon through in-stream heterotrophy and lateral inputs from tidal marshes (Cai 2011). While river inputs alone could support the observed levels of estuary outgassing for many estuaries, recent work in southeastern American estuaries suggests that the majority of CO2 comes from estuarine sources such as tidal floodplains and salt marshes instead of riverine sources (Jiang et al. 2008; Cai 2011). The same authors suggest that riverine inputs of organic carbon are remineralized in the estuarine plume or coastal waters, areas outside of the estuary itself. However, work in several smaller Northeastern American river estuaries showed that riverine sources of carbon dioxide were dominant, while the estuaries were smaller sources or even sometimes sinks of CO2 (Salisbury et al. 2008; Hunt et al. 2010).
Here, we present a study of the large, river-dominated macrotidal Kennebec estuary in Maine, which was surveyed 44 times from 2004 through 2008. This time-series of data allows us to examine the atmospheric CO2 exchange of the Kennebec estuary over monthly to annual time scales, and also allows us to examine the temporal variability of internal and riverine sources of CO2.
Methods
Study Site
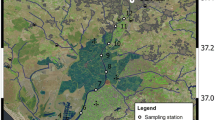
The Kennebec estuary is located along the central Maine (USA) coast (Fig. 1). It is supplied with freshwater from two large adjacent rivers: the more western Androscoggin and the eastern Kennebec. The combined Kennebec and Androscoggin river system, which drains 24,389 km2, represents one of the largest freshwater inputs to the Gulf of Maine system. Both the Androscoggin and Kennebec rivers are overwhelmingly forested (88.3 %), with a relatively small fraction of urban area (2.4 %), agricultural land (5.8 %), wetlands (3.4 %), and range (0.1 %, NOAA-CCMA). The geology of the river basins is characterized by till-covered bedrock with narrow valleys of stratified drift, with some fine-grained marine deposits in the lower watersheds. The rivers join at Merrymeeting Bay to form the Kennebec estuary, which extends about 35 km to the coast. The Kennebec’s tidal prism—the volume of water flowing into and out of the estuary on each tide—is large compared to inflows of Androscoggin and Kennebec river water (1 × 108 m3, or 1600 % of the average river discharge), and ebb currents are stronger than flood currents, especially during the spring freshet period (FitzGerald et al. 2000). During periods of low river flow the salinity = 2 isohaline can be located 30 km or more upstream of the estuary mouth—at Bath or even further north (Fig. 1)—while high river flows during the spring freshet can push the same isohaline completely out of the estuary and onto the adjacent shelf (Kistner and Pettigrew 2001). The residence time of water in the Kennebec estuary is estimated as 4 days (NOAA-CCMA). River end-member data were bucket samples collected from the center of bridges in Brunswick and Richmond, Maine for the Androscoggin and Kennebec rivers, respectively.
Map of the study site, located along the central Maine (USA) coast. The inset map shows the approximate location of the Kennebec estuary within the continental USA. River end-member sampling sites at Brunswick and Richmond are shown, as are sampling sites at Fort Popham and Fiddler’s Reach. The ten shaded regions outlined in black show the subregions of the estuary used for area-averaging calculations
Sampling and Analytical Methods
Surveys of the Kennebec estuary were conducted on a roughly monthly basis between September 24, 2004, and June 10, 2008, aboard the UNH research vessel R/V Gulf Challenger. A total of 44 surveys were conducted, with 22 of these on consecutive days while the ship docked overnight at Bath. Tidal state during the surveys covered the range from low tide to high, with most surveys conducted at mid-tide. Some single-day surveys turned around after sampling at Fiddler’s Reach (Fig. 1). A shipboard flow-through system was used to continuously measure physical and chemical properties of surface water. Temperature and salinity were determined by a Sea-bird SBE-45 thermosalinograph, while dissolved oxygen was measured with a Sea-bird SBE-43 sensor (Sea-bird electronics, Bellevue, WA). A temperature offset was observed between the sea surface temperature measured by the continuous-flow SBE-45 and that measured at the water surface by a SBE-37 thermosalinograph deployed as part of a profiling package. For each estuary survey, the average temperature offset between the continuous-flow and profiler sea-surface temperature was removed, to bring the continuous-flow temperature into agreement with in situ sea surface temperature. The SBE-45 thermosalinograph and SBE-43 oxygen sensor received annual manufacturer calibrations, but were not calibrated in the field against discrete measurements. The oxygen percent saturation was calculated according to Sea-bird Electronics Application Note 64 (Sea-bird Electronics 2013).
Flow to the shipboard flow-through system was also pumped to an equilibrator, similar to that described by Wanninkhof and Thoning (1993), but consisting of three Plexiglas chambers instead of a single chamber. Equilibrated air was drawn out of the third chamber, while ambient air was drawn into the first chamber and passed through the second and third chambers, equilibrating with the pumped water supply at each step. Equilibrated air was drawn at 100 mL/min through tubing containing a Nafion selectively permeable membrane (Perma Pure, Toms River NJ) with a counter-flowing stream of dry nitrogen, which dried the sample gas stream of water vapor. Due to the short run of tubing between the water source for both the continuous-flow system and the gas equilibrator, no water temperature difference was observed between that measured by the continuous-flow SBE-45 and the outflow from the equilibrator (measured with a handheld meter—YSI Yellow Springs, Ohio—manufacturer accuracy ±0.2 °C). Temperature from the continuous-flow SBE-45 was used in sea-surface temperature corrections during the calculation of pCO2. After drying, the sample was pumped to a non-dispersive infrared gas analyzer (Li-cor, LI-6262 or LI-840), which measured the molar fraction of carbon dioxide (xCO2) of the sample stream. The Li-cor was calibrated several times each survey with pure nitrogen (0 ppm CO2 molar fraction) and one span tank. Over the study period we employed a succession of span tanks containing a gas mixture with CO2 molar fraction between 819 and 851 ppm (Scott-Marin, Riverside, CA). Corrections of the data for water vapor pressure and sea surface temperature and conversion from xCO2 to the partial pressure of carbon dioxide (pCO2) were carried out according to standard methods (Dickson et al. 2007). Atmospheric pCO2 was periodically measured as well while the ship was underway. Ambient air was drawn from the ship’s bow through a length of tubing and pumped into the non-dispersive infrared gas analyzer described above. The estimated uncertainty of pCO2 measurements is ±3 μatm. All pCO2 data have been banked with the Carbon Dioxide Information Analysis Center (http://cdiac.ornl.gov/oceans/Coastal/unh_ts.html).
Discrete samples were collected from Niskin bottles in the Kennebec estuary, and transferred to sample bottles through silicone tubing to prevent bubbling. At the end-member sites, a bucket was lowered from a bridge upstream from each river’s most downstream dam, thoroughly soaked in the river water, and raised slowly to avoid promoting gas exchange. Samples for pH and total alkalinity (TAlk) were transferred without bubbling into 60-ml glass BOD bottles with greased stoppers. Beginning in 2006, dissolved inorganic carbon (DIC) was sampled from the same bottles. These were filled to leave less than 1 % headspace in the bottle, preserved with 0.1 ml of saturated mercuric chloride solution, and immediately cooled. Samples for TAlk and DIC were generally stored for several weeks before analysis, allowing for settling of particulate material, with the supernatant sample drawn for analysis. The in situ temperature of bucket samples was measured with the handheld YSI meter. DIC was measured first from each sample bottle, followed sequentially by pH and TAlk. Dissolved organic carbon (DOC) samples were collected according to JGOFS protocols (JGOFS 1996) into acid-washed high-density polyethylene bottles, and measured using a Shimadzu high temperature catalytic oxidation analyzer with chemiluminescent detection.
DIC of unfiltered water was determined using an automated analyzer built by Apollo SciTech (Bogart, GA). Immediately after opening the sample bottle, a digital syringe withdrew a small amount of sample (0.5 mL), acidified it with 10 % phosphoric acid and subsequently measured the evolved CO2 with a Li-Cor 6262 non-dispersive infrared gas analyzer (similar to the method described by Cai and Wang 1998). Certified seawater reference materials from Dr. A. Dickson were used to determine DIC concentration by preparing a calibration curve covering the range of DIC from 200 to 2000 μmol kg−1 (Dickson et al. 2003), with a resulting precision ranging from 0.05 to 0.5 % (or 0.1–10 μmol kg−1), with an average of ∼0.1 % (2 μmol kg−1).
TAlk and pH of unfiltered water were simultaneously measured by the same instrument, and thus pH and TAlk measurements are both based on the same pH electrode. The pH electrode used in the TAlk titration (Orion 3-Star, Thermo Fisher Inc.) was calibrated using three low ionic strength pH buffers certified on the U.S. National Bureau of Standards (NBS) scale to ±0.01, and the initial reading before the addition of acid titrant was taken as the sample pHNBS (pH on the NBS scale, hereafter simply referred to as pH).
TAlk was measured by Gran titration (Gran 1952) with 0.1 N HCl using an automated titrator. This method adds an initial aliquot of acid to the sample in an open cell, generally lowering the sample pHNBS below 3, and then adds subsequent aliquots of titrant until the pHNBS changes linearly with the volume of acid added. The TAlk endpoint is then obtained from linear regression of the change of pHNBS against the volume of acid added, according to a Gran transformation:
where GF is the resulting Gran Function, v is the volume of acid added to the sample, V o is the original sample volume, and pH is the pH value (in this case, on the NBS scale) measured after each successive addition of volume v. Multiple analyses of the Dickson reference material resulted in a calculated precision of this method of about 0.1 % (or ∼±2 μmol kg−1). The accuracy of the TAlk automated system, also calibrated with multiple batches of the Dickson CRM as discussed above, was ±3–4 μmol kg−1.
Non-Carbonate Alkalinity Correction
Recent work in New England rivers, including the Androscoggin and Kennebec rivers, indicates that non-carbonate species comprise a substantial portion of TAlk (Hunt et al. 2011). These non-carbonate species can include inorganic nutrients, metal complexes, and weak organic acids. Inclusion of these non-carbonate species in calculations to derive carbonate parameters can result in the overestimation of pCO2 and CO2 concentration. To account for this, we followed the methodology of Hunt et al. (2011) to derive TAlkDIC-pH from DIC and pHNBS measurements, with the resulting difference between measured TAlk and TAlkDIC-pH representing non-carbonate alkalinity (NC-Alk). All TAlk data presented in this work have been corrected for NC-Alk at levels that will be discussed later.
Air-Water CO2 Flux Estimation
The air–water flux of CO2 (F, in millimole per square meter per day) was calculated using the equation:
where k (centimeters per hour) represents the piston velocity of CO2, K 0 (in mole per cubic meter per atmosphere) is the solubility coefficient of CO2 at measured salinity and temperature, and pCO2water and pCO2air (in microatmosphere) are the measured partial pressures of CO2 in water and air, respectively. In this work, the term pCO2 will refer to pCO2water, unless otherwise indicated. Positive values of F indicate outgassing of CO2 from the water to the atmosphere. Due to a lack of appropriate equipment, the piston velocity was estimated from wind speed instead of measured directly. There has been much discussion of the best method to estimate the piston velocity of CO2 in estuaries, which is heavily dependent upon wind, but may also be affected by wave slope, surface films, rain, bottom-generated turbulence, surface turbulence, turbidity, and fetch limitation (Raymond and Cole 2001; Zappa et al. 2007; Borges et al. 2004a; Abril et al. 2009). A paper by Ho et al. (2011) examined the estimation of air–water gas exchange in the Hudson River, another northeastern USA estuary. The authors compared observed gas exchange rates measured using a dual tracer technique with those estimated from four wind-only k 600 parameterizations: Raymond and Cole (2001), Borges et al. (2004b), Ho et al. (2006)), and Jiang et al. (2008), finding that the Ho et al. (2006) parameterization most closely matched observed gas exchanges during two years of the study, while the Raymond and Cole (2001) parameterization most closely matched observations in a third year. The Jiang et al. (2008) and Borges et al. (2004b) parameterizations always overestimated the observed exchanges.
While discussion of gas flux results from the Kennebec estuary using the four parameterizations mentioned above will be presented later, fluxes in this work were estimated using the “tracers only” relationship from Raymond and Cole (2001) to calculate k 600:
where k 600 (in centimeter per hour) is the piston velocity at the Schmidt number of 600 and U is the wind speed (in meter per second). Hourly wind data were obtained from the Brunswick Air Station, just west of Bath.
To calculate area-averaged CO2 fluxes, the Kennebec estuary was broken into 10 segments (Fig. 1), although the upper segments were not accessible during some surveys, and are thus only included when data were available. The area-averaged flux was calculated from each segment as:
where F area-average is the area-averaged flux of all segments surveyed, F i is the average of all fluxes within segment i, and S i is the surface area of segment i.
Temperature-Normalized pCO2−
Influences upon estuarine pCO2 include factors both physical (water temperature, horizontal and vertical mixing) and biological (primary production and respiration). To examine the impact of seasonal water temperature changes and to contrast the influence of temperature to the aggregate influence of other factors, we calculated a temperature-normalized pCO2 at a temperature of 12 °C (pCO2obs@12 °C, Eq. 5), which is the mean area-averaged temperature measured in the Kennebec estuary during the surveys, according to the method of Takahashi et al. (2002):
where pCO2obs is the pCO2 at in situ temperature, T mean is the mean area-averaged temperature (12 °C), and T obs is the in situ temperature in degrees Celsius.
Changes in pCO2obs@12 °C represent the combined influences of horizontal mixing, vertical mixing, and biology if the water temperature remained at a constant 12 °C. In contrast, to calculate the effect of only temperature upon the mean annual pCO2, we again followed the method of Takahashi et al. (2002):
where pCO2mean@T obs is the area-averaged mean pCO2 at in situ temperature for each point during each survey and pCO2mean is the mean area-averaged pCO2.
Results
Hydrographic Conditions
Combined discharge data from the Androscoggin (USGS gage 1059000) and Kennebec (USGS gage 01049265) rivers is shown in Fig. 2, together with daily precipitation from the Portland International Jetport, located 50 km to the southwest of Bath. Generally, flow in the rivers is highest during two periods of the year: springtime, when snowmelt dominates the hydrograph, and fall, when rainfall from seasonal storms generates large amounts of rain and subsequent runoff, as seen in October and November 2005 and October 2006. Both the Androscoggin and Kennebec rivers trace their sources to large reservoirs, and are dammed at multiple points. These multiple impoundments increase the residence time of water in the rivers, and smooth out the hydrograph during larger precipitation events (Hunt et al. 2005). Surveys of Kennebec estuary pCO2 were conducted over a wide range of river flow conditions (Fig. 2). The highest-flow survey was conducted October 18, 2005, during a fall storm, while the lowest-flow survey was conducted July 19, 2007. The average combined river flow on days when surveys were conducted (485 m3 s−1) was lower than the average of all daily river flow measurements taken over the time period of this study (566 m3 s−1). For 2005, 2006 and 2007, the 3 years of the survey period with relatively complete temporal coverage (at least 11 transects per year), the average annual combined discharge of the rivers was 713, 588, and 501 m3s−1, respectively. Long-term flow data from the USGS indicates that the combined average daily discharge is 459 m3s−1.
Combined average daily discharge from the USGS gages 1059000 (Androscoggin river) and 01049265 (Kennebec river, solid line). Filled circles indicate dates of Kennebec estuary surveys, while filled bars show NOAA National Climate Data Center daily precipitation from the Portland International Jetport, 50 km southwest of Bath
Salinity at the top of the estuary transect (Figs. 1 and 3) was below 1.0 for 32 of the 44 surveys. Months where salinity at the uppermost transect reach was higher than 1.0 were typically the low-flow summer and fall months of August, September, and October (Table 2). In general, however, the surveys were able to satisfactorily cover the full salinity gradient, perhaps with the exception of October 18, 2005, when salinity at Fort Popham was only about 15. Annual, area-averaged salinity for the whole Kennebec estuary was lower in 2005 (11.4) than in 2006 (12.7) or 2007 (17.9, σ = 5.9, 3.3, and 6.2 respectively), which agrees with the annual differences in combined river discharge.
Variations of Kennebec estuary salinity, water temperature, pCO2 and CO2 flux over the sampling period from the estuary mouth (43.74° latitude at Fort Popham) to Fiddler’s Reach (43.88° latitude). Circles on the x-axis mark dates when the estuary was surveyed. Bilinear interpolation was used between surveys
Water temperature at the upper transect reach was more variable than at Fort Popham (Fig. 3). During the winter months, water temperature was lower at the uppermost transect reach than Fort Popham, while during the summer months, the uppermost transect reach was warmer than Fort Popham. For some time in the spring (usually April or May) and in the fall (usually October), the water temperature was essentially the same along the entire transect.
River Chemistry
The Kennebec and Androscoggin rivers, like many rivers in the New England region, have lower TAlk and are more acidic (Table 1) than other rivers around the USA (Hunt et al. 2011; Salisbury et al. 2008). On average, half of the alkalinity leaving the rivers is composed of non-carbonate species (Hunt et al. 2011). This may be explained in part by the presence of organic acids as a component of DOC (Eshleman and Hemond 1985; Cai et al. 1998), although borate, inorganic nutrients, and metal complexes may be contributors to NC-Alk as well. Concentrations of DOC in the Androscoggin and Kennebec rivers are of the same order of magnitude as TAlk (Table 1), and the presence of organic acids in the DOC pool would contribute to both the lower pH and higher levels of NC-Alk. DIC was also relatively low for northeastern rivers (Raymond et al. 2004), and pCO2 derived from DIC and pH river end-member measurements was both always above atmospheric levels and always above the highest pCO2 measured in the Kennebec estuary (Table 2).
Kennebec Estuary pCO2
In general, Kennebec estuary pCO2 decreased from the river to the ocean, and followed a seasonal pattern of lower pCO2 in the winter and spring and higher pCO2 in the summer and fall (Fig. 3). For the period of this study, the area-averaged pCO2 in the Kennebec estuary was 558 μatm (σ = 167 μatm), while the average observed pCO2air was 383 μatm (σ = 8 μatm). The lowest measured pCO2 was 203 μatm (at salinity 9.78) in April 2008, while the highest pCO2 was 1,771 μatm (at salinity 1.17) in June 2005. While an overall seasonal pattern of pCO2 was observed, storm events had a dramatic effect on pCO2 on shorter time scales. The two surveys with highest pCO2 (Fig. 3) were conducted on June 29, 2005, (1,725 μatm) and June 28, 2006 (1,771 μatm). River discharge on the days of these surveys was not especially high (Fig. 2), but both surveys followed very large storm events which released large amounts of river water into the estuary. A survey of three New Hampshire estuaries located approximately 150 km from the Kennebec estuary, which also experienced the same large June 2006 storm event, showed elevated pCO2 as well (Hunt et al. 2010). Storm events, especially during the warmer months when the ground is not frozen, can presumably flush out soil and groundwater enriched in CO2 (Paquay et al. 2007) as well as large amounts of labile particulate and dissolved organic matter, which could support bacterial respiration and CO2 production in the estuary (Abril et al. 2000; Abril and Borges 2004). The elevated pCO2 observed in the June 2005 and 2006 surveys was more than twice the pCO2 levels in June 2007 (highest pCO2: 780 μatm) and June 2008 (highest pCO2: 604 μatm) when river discharge was at typical low-flow summer levels (Fig. 2).
Data from each survey was binned by salinity and averaged every five salinity units (Fig. 4). The lowest salinity bin (salinity 0–5) never had pCO2 below atmospheric levels, and pCO2 ranged as high as 1,711 μatm (in June 2005) for this bin, with a mean pCO2 of 888 μatm (σ = 304 μatm). At the high-salinity end of the estuary (25–30 salinity), the pCO2 ranged from 199 to 608 μatm, with a mean pCO2 of 392 μatm (σ = 78 μatm). The bin with pCO2 most closely resembling the whole-estuary area-averaged pCO2 was salinity 10-15, as illustrated by the difference between the binned pCO2 and the estuary-average pCO2 (Figs. 4 and 5). It is noteworthy that the difference in pCO2 between salinity bins was always highest between the 0–5 and 5–10 salinity bins, except in the month of September when the 0–5 salinity pCO2 values decreased from 1,130 to 891 μatm. This suggests that the largest changes in CO2 along the salinity gradient occur at low salinities, when river water is initially mixed with water from the estuary.
Monthly mean values of area-averaged pCO2 (open circles), climatology of pCO2 after 60-day smooth (solid line), monthly mean of area-averaged oxygen saturation (plus signs), and climatology of the percent saturation of oxygen (dashed line), constructed using the same smoothing method as area-averaged pCO2
To examine the annual pattern of CO2 in the Kennebec estuary, we constructed a yearly climatology. We combined the area-averaged pCO2 values into monthly averages, repeated the annual cycle of monthly values three times (to obtain reasonable boundary values for January and December), ran a 60-day Matlab smooth function over the resulting timeseries (‘smooth’, The MathWorks, Inc., Natick MA), then took the middle year as the final climatology (Fig. 5). The resulting line shows a clear drop in pCO2 during the late winter and early spring (February and March), a steady rise in pCO2 through the spring and summer, more or less constant pCO2 over the fall months, then a sharp decline in pCO2 through November and December. Opposing trends were seen in the saturation of dissolved oxygen (Fig. 5), which rose through the winter to a peak in April (a month later than the lowest pCO2 in March), then dropped through the late spring and summer to a low in September (a month before the peak pCO2 in October). This seasonal coupling between pCO2 and dissolved oxygen indicates that biological activity contributes noticeably to overall pCO2 in the Kennebec estuary. The cycle of pCO2 in Fig. 5 also bears a strong resemblance to the annual cycle observed at inner shelf coastal stations in the Gulf of Maine (Vandemark et al. 2011), as well as the BATS site near Bermuda (Takahashi et al. 2002) and a Spanish coastal site (Ribas-Ribas et al. 2011).
The solubility of CO2 decreases with increasing water temperature (Carroll et al. 1991), and in the New England region warmer water temperatures in the summer and fall increase pCO2, while cooler temperatures in the winter and spring months drive pCO2 lower. If water temperature was the sole control upon pCO2, the pCO2obs@12 °C should be fairly constant over the course of the year. We repeated the above process to construct salinity-binned climatologies of temperature-normalized pCO2 (pCO2obs@12 °C, Fig. 6). The annual patterns of pCO2obs@12 °C reflect how the observed pCO2 would change if the water temperature in the estuary were held at a constant 12 °C, and differ from those of pCO2 at in situ temperature (Fig. 4). Seasonal patterns of pCO2obs@12 °C are markedly different from the cycle of pCO2 at in situ temperature, and differ between salinity bins. The highest pCO2obs@12 °C was generally in winter (December or January), followed by a steady decrease similar to that seen at in situ temperature. For the lowest-salinity bin, this decrease continued through the spring and summer, to an annual minimum in September. For all other salinity bins the annual pCO2obs@12 °C minimum was much earlier—in April or May. Mid-salinity bins (salinities 5–10, 10–15, 15–20, and 20–25) all had a secondary pCO2obs@12 °C maximum in August. While pCO2obs@12 °C rose sharply between September and October in the 0–5 salinity bin, pCO2obs@12 °C dropped in the same time period in the higher-salinity bins. In all salinity bins, pCO2obs@12 °C rose through late fall back to the annual winter maximum. The observed difference between the climatologies of pCO2 and pCO2obs@12 °C indicate that temperature variability is one important process controlling seasonal pCO2 changes. In addition to temperature, pCO2 is influenced by the seasonality of river/ocean mixing in the estuary and net community productivity.
Salinity-binned climatologies of temperature-normalized observed pCO2 at 12 °C (pCO2obs@12 °C, solid line) and the mean, area-averaged pCO2 at in situ temperature through the year (pCO2mean@Tobs, dashed line). The mean pCO2 values for each bin were 857, 619, 471, 424, 407, and 396 μatm for salinities 0–5, 5–10, 10–15, 15–20, 20–25, and 25–30, respectively. The dashed vertical arrow in the salinity 0–5 panel shows the magnitude of ∆pCO2temp, while the solid vertical arrow in the same panel shows ∆pCO2bio
Air-Water CO2 Fluxes
Estimates of k 600 in the Kennebec estuary using the Raymond and Cole (2001) parameterization resulted in area-averaged CO2 fluxes (average = 8.6 mmol m−2 day−1) that were higher than those calculated according to Ho et al. (2004, average = 6.3 mmol m−2 day−1), but lower than those from Jiang et al. (2008), average = 12.4 mmol m−2 day−1 or Borges et al. (2004b), average = 16.1. Paired t tests showed that the average fluxes from all four parameterizations were significantly different (p < 0.01). As no site-specific parameterization of gas exchange is available for the Kennebec estuary, we chose to use the Raymond and Cole (2001) parameterization, as it demonstrated reasonable results in the Hudson estuary (Ho et al. 2011), and represents a flux estimate bracketed by other published estimates. Thus, all further CO2 fluxes presented in this work were calculated using the Raymond and Cole (2001) parameterization.
Area-averaged fluxes in the Kennebec estuary were mostly positive in sign, meaning that CO2 was released from the estuary to the atmosphere. The Kennebec estuary released CO2 to the atmosphere in 38 of the 44 surveys performed. There was no significant correlation observed between tidal height and area-averaged CO2 flux (r 2 = 0.0024), indicating that other factors controlled CO2 fluxes more than the tide. The overall area-averaged CO2 flux from the estuary was 8.6 mmol m−2 day−1 (σ = 8.9 mmol m−2 day−1). The flux of CO2 decreased in late winter (February) to a minimum in early spring (March), then increased rapidly to an annual peak in April, before leveling out and gradually decreasing through the summer and fall to another minimum in December. The lowest value of area-averaged CO2 flux was on February 23, 2006 (−11.5 mmol m−2 day−1), while the largest area-averaged flux was on April 19, 2005 (31.7 mmol m−2 day−1). When the daily fluxes provided by the climatology are summed and multiplied by the total surface area of the estuary (2 × 107 m2), the result is an annual flux of 7.2 × 107 mol C, or 3,160 metric tons of CO2.
Temperature played a role in suppressing the flux of CO2 on an annual basis, as the average CO2 flux estimated at a constant temperature of 12 °C (11.4 mmol m−2 day−1) was well above the annual average flux calculated at in situ temperature (8.6 mmol m−2 day−1). However, the large CO2 flux decrease between January and March (Fig. 7), and the subsequent CO2 flux increase from March to April, appear to be caused by factors apart from temperature, as does the CO2 flux increase later in the year (November and December).
Salinity-binned fluxes (Fig. 8) exhibited similar monthly trends, apart from the 0–5 salinity data. This lowest salinity bin mirrors the monthly pattern of overall CO2 flux in the estuary depicted in Fig. 7, with a decline in CO2 flux in March and a sharp peak in CO2 flux in April. Despite the same wind data being used in all salinity bins, the higher salinity bins show the spring decline a month later, in April, followed by a steady increase in CO2 flux until August or September. It is apparent from the binned flux data that the 0–5 salinity bin exerts a strong influence on overall fluxes from the estuary. The area of the estuary represented by the 0–5 salinity bin, calculated assuming a consistent average channel width and averaging the latitudinal distribution of salinity, varies widely due to seasonal and episodic changes in river discharge (Fig. 2), ranging from 0 to 60 % of the estuary area, with a mean of 18 % (σ = 17 %) of the estuary area. Thus, especially in the spring months of March and April when river discharge is high and estuary salinity is low, CO2 fluxes from the 0–5 salinity bin may play a disproportionally large role in the overall flux of CO2 from the estuary.
Discussion
The pCO2 in estuaries is controlled by inputs of CO2 from rivers, the ocean, and within the estuary itself, together with water temperature, horizontal and vertical mixing, and net community productivity. In the following sections, we will discuss the effects of changing water temperature and inputs of CO2 from river and within-estuary sources, and compare results from the Kennebec estuary to some other estuaries. It is important to note that the data presented here, particularly CO2 fluxes, represent the surveyed portion of the Kennebec estuary, not the entire estuary. Merrymeeting Bay (Fig. 1) is the upper tidal portion of the estuary, with a surface area more than twice that of the lower portion of the estuary we surveyed. So, although the surveyed portion of the estuary represented almost the entire salinity gradient (Table 1), a rigorous definition such as that of Perillo (1995), “a semi-enclosed coastal body of water that extends to the effective limit of tidal influence” would include Merrymeeting Bay and some lower portion of the Androscoggin and Kennebec rivers; thus total fluxes, as well as average pCO2 and area-specific CO2 fluxes (i.e., in millimole per square meter per day), are underestimates. Indeed, since the Merrymeeting Bay portion of the estuary would fall into the 0–5 salinity bin- the bin with highest pCO2− estimates of CO2 flux from the whole Kennebec estuary, including Merrymeeting Bay, are likely to increase by a factor of three or more.
Changes in water temperature in a northern latitude estuary occur on a seasonal basis, resulting in higher pCO2 in the summer and lower pCO2 in the winter. The temperature of the Androscoggin and Kennebec rivers varies from a low of nearly 0 °C to a high of over 25 °C, while the ocean end-member water temperature ranges from 1.4 to 16 °C. The timing of maximum and minimum temperatures also differs between the rivers and ocean end-member. River temperature reaches its minimum in January and February, while the surface ocean temperature minimum is later, in April. Additionally, the rivers warm up faster in the spring, so that by May the rivers are warmer than the ocean end-member. Maximum river water temperature is in July or August, while the maximum ocean end-member temperature occurs in September or October. The Androscoggin and Kennebec rivers spend much of the winter covered in ice, usually from December until spring snowmelt in March or April. When the spring snowpack melts, there is a large pulse of very cold freshwater discharged to the estuary, which lowers water temperature and pCO2 and also delivers inorganic nutrients (Oczkowski et al. 2006) and presumably DOC to the estuary.
Temperature affected both pCO2 and CO2 flux in the Kennebec estuary, as seen by the differences between temperature-normalized pCO2 and CO2 flux and the observed patterns at in situ temperatures (Figs. 4, 5, and 6). While the observed pCO2 is lowest in March or April, the lowest pCO2obs@12 °C occurs in May or later in all salinity bins. This suggests that cold inputs of river water in the spring act to lower the pCO2 in the estuary. This can also be seen in Fig. 4, since the lowest pCO2 in the 0–5 salinity bin at in situ temperature occurred in March, while the lowest pCO2 in the other salinity bins was observed later, in April. Increasing water temperature in the estuary from March through July was also responsible for the rise of pCO2 through the same time period. The yearly progression of temperature-normalized pCO2 shows that seasonal water temperature changes do not explain all observed variability of Kennebec estuary pCO2. Temperature-normalized pCO2 decreased during the spring as in situ pCO2 also decreased, but while in situ pCO2 rose from May to August due to increasing water temperatures, pCO2obs@12 °C in the 0–5 salinity bin continued decreasing. This suggests two possible scenarios: one, that decreasing river flow through the spring into the summer resulted in lower inputs of terrestrial DIC and organic carbon to the estuary, or two, that in situ primary production at low salinities through the spring into mid-summer drew down the pCO2, while increased respiration through late summer and the fall raised the pCO2. Patterns of pCO2obs@12 °C in the other salinity bins more closely resembled those at in situ temperature, but with the annual pCO2 minimum shifted a month or two later. Takahashi et al. (2002) calculated the effect of biology on surface water pCO2 as:
where (pCO2obs@12 °C)max and (pCO2obs@12 °C)min are the maximum and minimum values of pCO2obs@12 °C in the time-series, respectively. Correspondingly, we calculated the effect of changing temperature on the mean surface pCO2 value in each salinity bin (pCO2mean@Tobs) according to Takahashi et al. (2002):
The value of ∆pCO2bio also includes the effects of horizontal and vertical mixing for the Kennebec estuary. We believe the effects of vertical mixing to be relatively small in the Kennebec estuary; however, horizontal mixing appears to contribute to ∆pCO2bio, especially in the 0–5 salinity bin as the pCO2obs@12 °C continues to decrease as river inputs decrease through the late spring into the summer and fall. The difference between ∆pCO2temp and ∆pCO2bio (T − B, Table 3), together with the ratio of ∆pCO2temp/∆pCO2bio (T/B, Table 3) yield the relative importance of temperature and biology/mixing on the Kennebec estuary as a whole; in this case temperature changes have about twice the effect of biology/mixing on pCO2 for salinities 0–15 (T/B ∼ 2, Table 3), while for salinities greater than 15 the T/B drops steadily to a value of 1.0. Thus, while both ∆pCO2temp and ∆pCO2bio decrease with increasing salinity, ∆pCO2temp decreases more. This decreasing influence of temperature with increasing salinity is reasonable, as the range of river water temperatures is greater than that at the ocean end-member. The ∆pCO2bio decreases with increasing salinity to a minimum in the 20–25 salinity bin, perhaps indicating a decreasing influence of river CO2 input to salinity 20, followed by an enhanced ocean biologic CO2 signal at salinities greater than 25 due to upwelling or some other factor. In Takahashi et al. (2002), the temperature effect in the Gulf of Maine exceeded the effect of biology, indicating that the effects of biology/mixing at the mouth of the Kennebec estuary are stronger than those generally observed in the Gulf of Maine.
For 2005, 2006, and 2007, the 3 years with the best survey coverage, the mean area-averaged CO2 fluxes were 13.9, 5.4, and 5.8 mmol m−2 day−1, respectively (σ = 9, 9 and 7 mmol m−2 day−1, respectively). The year-to-year variation in annual mean CO2 flux may be partly explained by river discharge. Annual river discharge in 2005 was the highest measured for the Kennebec river in 24 years, and the second-highest in the Androscoggin river for 82 years of record. Elevated river discharge delivers large fluxes of CO2 and DOC to estuaries, resulting in elevated CO2 fluxes from the estuary (e.g., Hunt et al. 2011). Additionally, elevated river flow in 2005 reduced the overall surface salinity in the estuary, and since lower salinity is generally associated with high pCO2 in the Kennebec estuary, the higher proportion of low-salinity waters in the estuary resulted in elevated fluxes of CO2 from the estuary as a whole.
The average annual flux of CO2 to the atmosphere from the Kennebec estuary was 3.5 mol m−2 year−1 (σ = 1.0 mol m−2 year−1) over the study period. A review of estuary CO2 fluxes (Borges and Abril 2011) provides a reference for CO2 fluxes from estuaries similar to the Kennebec, with the range of fluxes from “tidal systems and embayments” reported as 1.1–76 mol m−2 year−1. This places the CO2 flux from the Kennebec estuary on the low end of the range. The Kennebec flux is consistent with data from five other estuaries in the region, which only range from 1.1 to 4.0 mol m−2 year−1 (Raymond and Hopkinson 2003; Hunt et al. 2010). One possible explanation as to why the Kennebec and other New England estuaries seem to have lower pCO2 and CO2 fluxes than their global counterparts is a lack of CO2 input from the estuaries themselves. Some estuaries have been shown to enhance CO2 levels (Cai 2011; Gupta et al. 2009; Jiang et al. 2008; Raymond et al. 2000). In fact, some researchers have observed that the majority of CO2 in the estuary is produced internally from the lateral transport of DIC produced by microbial organic matter respiration in tidal marshes or mangroves which fringe the estuary (Cai 2011; Cai and Wang 1998, 2004), although CO2 production via respiration in the water column or sediments is also a potential contributor (Raymond et al. 2000). The Kennebec estuary does have some tidal marshes, particularly around smaller tributaries, but overall they are not a large presence along the steep, rocky shoreline. In other New England estuaries the majority of CO2 came from the rivers, and during the spring (and fall to a lesser extent) the estuarine areas removed CO2 (Salisbury et al. 2008; Hunt et al. 2010). Another possible explanation for the seeming lower CO2 fluxes from the Kennebec estuary as compared to other global estuaries is that fluxes from Merrymeeting Bay, which covers a large amount of area at the freshwater end of the estuary, were not measured and would presumably reflect high pCO2 from river inputs, raising the overall CO2 flux from the estuary as a whole.
To examine the inputs of CO2 from river and estuary sources, we followed the approach of Jiang et al. (2008), who partitioned dissolved CO2 concentrations into ocean, river and estuary sources according to conservatively mixed salinity. Specifically, the CO2 contribution from the estuary (Δ[CO2]estuary) to in situ estuarine dissolved CO2 ([CO2]i) is calculated as the difference between [CO2]i and the CO2 concentration if the ocean and river end-members mix conservatively ([CO2]mixing w/R). The CO2 concentration of river input (Δ[CO2]river) was calculated as:
where [CO2]mixing w/R is the CO2 concentration if the ocean and river end-members mix conservatively, and [CO2]mixing w/O is the CO2 concentration if the ocean end-member is diluted by fresh water with a CO2 of zero. Conservative mixing of TAlk and DIC (calculated from either TAlk and pH or TAlk and pCO2, since DIC was not measured in 2004 and 2005) was used to calculate the mixing curves. Observed oceanic concentrations of DIC and TAlk were conservative with salinity, and there was no evidence of a seasonal change in oceanic DIC or TAlk concentration with respect to salinity. Inputs from the river end-members were controlled by river flow, with the highest fluxes of TAlk and DIC observed during spring snowmelt and storms in the late fall (Fig. 2).
The results of these analyses showed that both the river and estuary made positive contributions to CO2 in the Kennebec estuary in all months (Fig. 9). The river was generally the dominant source of CO2 to the estuary- the only month that the estuary input of CO2 exceeded that of the river was June (56 % of CO2 was from estuary). Overall, the monthly estuary contribution of CO2 ranged from 14 to 56 %, with an average contribution of 33 % (σ = 12 %). On an annual basis, the average contributions of the Kennebec estuary to CO2 were indistinguishable at 36, 32 and 38 % in 2005, 2006, and 2007, respectively (σ = 38, 41, 47 %, respectively). Both the estuary and river contributed the most CO2 in 2005 (14.4 and 8.2 μmol L−1, respectively), and the least CO2 in 2007 (6.6 and 4.1 μmol L−1, respectively). This is in contrast to estuaries in the Southeastern USA, such as the river-dominated Satilla (Cai and Wang 1998) and Altamaha (Jiang et al. 2008) river estuaries and the marsh-dominated Sapelo and Doboy sounds (Jiang et al. 2008). In all these Southeastern systems, a seasonal pattern of increased marsh influence has been discussed, from low marsh inputs in spring, increasing inputs through summer into fall and early winter, then decreasing inputs through winter back into spring (Cai 2011). However, the input of CO2 from the Kennebec estuary does not appear to show a seasonal progression that would be associated with the growth and die-off of marsh plants.
Our observations in the Kennebec estuary suggest a system whose main CO2 source is the degassing of river-borne DIC, with in-channel heterotrophy being a secondary input, and marsh inputs representing a small contributor of CO2. A study of 11 European and American estuaries calculated the median contribution of riverine DIC to estuary CO2 degassing at 10 % of total CO2 release, with heterotrophic activity accounting for the remaining 90 % of CO2 release, and riverine contributions increasing with decreasing estuary residence time (Borges et al. 2006). This seems consistent with our observations in the Kennebec estuary, as the Kennebec residence time is quite short (4 days, NOAA-CCMA) and the riverine CO2 contribution is relatively high as discussed above.
The balance of river and marsh DIC contributions to global estuaries remains unclear. Coastal marshes are generally found in northern temperate areas from about 30°N to 65°N, with mangroves becoming dominant south of 30°N (Ibanez et al. 2012), although the seagrasses which typically dominate marsh vegetation can also be found throughout the tropics (UNEP-WCMC 2005). Overall, seagrass and salt marsh together cover an order of magnitude more area than mangroves (158 × 106 and 15 × 106 ha, respectively, Duarte et al. 2008). There are large areas of the global coastline, notably the western African and South American coasts and higher-latitude coasts, which do not support documented marsh or mangrove areas (UNEP-WCMC 2005). Additionally, marsh habitat is being lost at a rapid pace- estimated to be up to 2 % per year- with over 30 % of total habitat lost since the 1940s (Duarte et al. 2008). As discussed above, CO2 release from numerous estuaries has been shown to be marsh-dominated; however, we speculate that higher-latitude temperate areas such as the Kennebec estuary may be more river-influenced, presenting a scaling challenge to integrating studies of individual estuaries into a global whole. Finally, as marsh areas continue to decline, riverine inputs of DIC could begin to dominate in more systems.
Conclusions
The seasonal cycle of Kennebec estuary pCO2 displayed a pattern of decreased pCO2 in late winter and early spring, coincident with colder water temperatures and the annual spring phytoplankton bloom, followed by increasing pCO2 through late spring and summer, then decreasing pCO2 through the fall and early winter. The Kennebec estuary was a net source of CO2 to the atmosphere, and monthly fluxes of CO2 from the Kennebec estuary were positive except for the month of March as the spring bloom drew down CO2. Warmer water temperatures in New England promote increased pCO2 in the summer and fall months, while cooler temperatures lead to decreased pCO2 in the winter and spring. The yearly progression of temperature-normalized pCO2 showed that water temperature was not the only factor controlling Kennebec estuary pCO2, as pCO2obs@12 °C decreased during the spring as did in situ pCO2, but unlike in situ pCO2 continued decreasing into the late spring or even summer, well past the time when in situ pCO2 was rising due to increasing water temperatures. Overall, temperature changes accounted for more of the pCO2 variability than biological activity and mixing. The balance between the influences of biology and mixing is not clear, but both appear to be important processes in the Kennebec estuary. Storm events, which generated large amounts of rainfall and subsequent river discharge to the estuary, were shown to produce elevated pCO2 in the estuary in June in two consecutive years, and these events may affect overall annual rates of CO2 efflux from estuaries. River discharge also appears to influences the CO2 flux from the estuary on an annual scale. The highest annual average flux of CO2 from the Kennebec estuary to the atmosphere was in 2005, the year with the highest annual river discharge and lowest annual average salinity. While both the estuary and river contributed to overall estuary CO2, contributions from the river were twice the magnitude of those from the estuary. The monthly estuary contribution of CO2 ranged from 14 to 56 %, with an average contribution of 33 %. There was no discernible seasonality in estuary or river contributions to overall estuary CO2. Future work should extend the findings presented here to the coastal ocean, and examine how inorganic and organic carbon releases from the Kennebec estuary spread into the coastal Gulf of Maine, as well as the persistence and fate of these fluxes.
References
Abril G. & A.V. Borges 2004 Carbon dioxide and methane emissions from estuaries. In Environmental Science Series: Greenhouse gases emissions from natural environments and hydroelectric reservoirs: fluxes and processes, eds. A. Tremblay, L. Varfalvy, C. Roehm and M. Garneau (Eds), Chapter 7, pp. 187–207, 730 pages. Berlin, Springer
Abril, G., H. Etcheber, A.V. Borges, and M. Frankignoulle. 2000. Excess atmospheric carbon dioxide transported into the Scheldt estuary. Compte Rendus de l’Academie des Sciences de Paris, Sciences de la Terre et des planets 300: 761–768.
Abril, G., M.V. Commarieu, A. Sottolichio, P. Bretel, and F. Guerin. 2009. Turbidity limits gas exchange in a large macrotidal estuary. Estuarine, Coastal and Shelf Science 83: 342–348.
Borges, A.V. 2005. Do We have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries and Coasts 28: 3–27.
Borges, A.V., and G. Abril. 2011. Carbon dioxide and methane dynamic in estuaries. In Treatise on estuarine and coastal science, vol. 5, ed. E. Wolanski and D.S. McLusky, 119–161. Waltham: Academic Press.
Borges, A.V., B. Delille, L.-S. Schiettecatte, F. Gazeau, G. Abril, and M. Frankignoulle. 2004a. Gas transfer velocities of CO2 in three European estuaries (Randers Fjord, Scheldt, and Thames). Limnology and Oceanography 49: 1630–1641.
Borges, A.V., J.P. Vanderborght, L.S. Schiettecatte, F. Gazeau, S. Ferron-Smith, B. Delille, and M. Frankignoulle. 2004b. Variability of the gas transfer velocity of CO2 in a macrotidal estuary. Estuaries 27: 593–603.
Borges, A.V., L.-S. Schiettecatte, G. Abril, B. Delille, and F. Gazeau. 2006. Carbon dioxide in European coastal waters. Estuarine, Coastal and Shelf Science 70: 375–387.
Cai, W.-J. 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks of sites of terrestrial carbon incineration? Annual Review of Marine Science 3: 123–145.
Cai, W.-J., and Y. Wang. 1998. The chemistry, fluxes and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnology and Oceanography 43: 657–668.
Cai, W.-J., and Y. Wang. 2004. Carbon dioxide degassing and inorganic carbon export from a marsh-dominated estuary (the Duplin River): a marsh CO2 pump. Limnology and Oceanography 49: 341–354.
Cai, W.-J., Y. Wang, and R.E. Hodson. 1998. Acid-base properties of dissolved organic matter in the estuarine waters of Georgia, USA. Geochimica et Cosmochimica Acta 62: 473–483.
Cai, W.-J., M. Dai, and Y. Wang. 2006. Air-sea exchange of carbon dioxide in ocean margins: a province-based synthesis. Geophysical Research Letters 33, L12603.
Carroll, J.J., J.D. Slupsky, and A.E. Mather. 1991. The solubility of carbon dioxide in water at low pressure. Journal of Physical and Chemical Reference Data 20(6): 1201–1209.
Dickson, A.G., J.D. Afghan, and G.C. Anderson. 2003. Reference materials for oceanic CO2 analysis: a method for the certification of total alkalinity. Marine Chemistry 80: 185–197.
Dickson, A.G., Sabine, C.L. and J.R. Christian (Eds.). 2007. Guide to best practices for ocean CO2 measurements. PICES Special Publication 3, 191 pp.
Duarte, C.M., W.C. Dennison, R.J.W. Orth, and T.J.B. Carruthers. 2008. The charisma of coastal ecosystems: addressing the imbalance. Estuaries and Coasts 31: 233–238.
Eshleman, K.N., and H.F. Hemond. 1985. The role of organic acids in the acid-base status of surface waters at Bickford Watershed, Massachusetts. Water Resources Research 10: 1503–1510.
FitzGerald, D.M., I.V. Buynevich, M.S. Fenster, and P.A. McKinlay. 2000. Sand dynamics at the mouth of a rock-bound, tide-dominated estuary. Sed Geo 131: 25–49.
Gattuso, J.-P., M. Frankignoulle, and R. Wollast. 1998. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annual Review of Ecology and Systematics 29: 405–434.
Gran, G. 1952. Determination of the equivalence point in potentiometric titrations. Part II. Analyst 77: 661–671.
Gupta, G.V.M., V.V.S.S. Sarma, R.S. Robin, M.J. Kumar, M. Rakesh, and B.R. Subramanian. 2008. Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO2 in a tropical coastal lagoon (Chilka Lake, India). Biogeochemistry 87: 265–285.
Gupta, G.V.M., S.D. Thottathil, K.K. Balachandran, N.V. Madhu, P. Madeswaran, and S. Nair. 2009. CO2 supersaturation and net heterotrophy in a tropical estuary (Cochin, India): influence of anthropogenic effect. Ecosystems 12: 1145–1157.
Ho, D.T., C.S. Law, M.J. Smith, P. Schlosser, M. Harvey, and P. Hill. 2006. Measurements of air-sea gas exchange at high wind speeds in the Southern Ocean: implications for global parameterizations. Geophysical Research Letters 33, L16611.
Ho, D.T., P. Schlosser, and P.M. Orton. 2011. On factors controlling air-water gas exchange in a large tidal river. Estuaries and Coasts 34: 1103–1116.
Hunt, C.W., T. Loder, and C. Vörösmarty. 2005. Spatial and temporal patterns of inorganic nutrient concentrations in the Androscoggin and Kennebec Rivers, Maine. Water, Air, and Soil Pollution 163: 303–323.
Hunt, C.W., J.E. Salisbury, D. Vandemark, and W. McGillis. 2010. Contrasting carbon dioxide inputs and exchanges in three adjacent New England estuaries. Estuaries and Coasts. doi:10.1007/s12237-010-9299-9.
Hunt, C.W., J.E. Salisbury, and D. Vandemark. 2011. Contributions on non-carbonate anions to total alkalinity and overestimation of pCO2 in New England and New Brunswick rivers. Biogeosciences. doi:10.5194/bg-8-3069-2011.
Ibanez, C., J.T. Morris, I.A. Mendelssohn, and J.W. Day. 2012. Coastal Marshes. In Estuarine ecology, 2nd ed, ed. J.W. Day, W.M. Kemp, A. Yáñez-Arancibia, and B.C. Crump, 129–164. Hoboken: Wiley-Blackwell.
JGOFS 1996. Protocols for the Joint Global Ocean Flux studies (JGOFS) core measurements SCOR/ICSU/IOC. p. 170.
Jiang, L.-Q., W.-J. Cai, and Y. Wang. 2008. A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries. Limnology and Oceanography 53: 2603–2615.
Kistner, D.A., and N.R. Pettigrew. 2001. A variable turbidity maximum in the Kennebec Estuary, Maine. Estuaries 24: 680–687.
Laruelle, G.G., H.H. Dürr, C.P. Slomp, and A.V. Borges. 2010. Evaluation of sinks and sources of CO2 in the global coastal ocean using a spatially‐explicit typology of estuaries and continental shelves. Geophysical Research Letters 37, LI5607. doi:10.1029/2010GL043691.
NOAA-CCMA. Kennebec Estuary Summary: http://ccma.nos.noaa.gov/stressors/pollution/eutrophication/eutrocards/kennebec.pdf. Accessed 12 June 2013.
Oczkowski, A.J., B.A. Pellerin, C.W. Hunt, W.W. Wollheim, C.J. Vorosmarty, and T.C. Loder. 2006. The role of snowmelt and spring rainfall in inorganic nutrient fluxes from a large temperate watershed, the Androscoggin River basin (Maine and New Hampshire). Biogeochemistry 80: 191–203.
Paquay, F.S., F.T. Mackenzie, and A.V. Borges. 2007. Carbon dioxide dynamics in rivers and coastal waters of the “big island” of Hawaii, USA, during baseline and heavy rain conditions. Aquatic Geochemistry 13: 1–18.
Perillo, G.M.E. 1995. Definitions and geomorphological classifications of estuaries. In Geomorphology and Sedimentology of Estuaries, ed. G.M.E. Perillo, 17–47. Amsterdam: Elsevier.
Raymond, P.A., and J.J. Cole. 2001. Gas exchange in rivers and estuaries: choosing a gas transfer velocity. Estuaries 24: 312–317.
Raymond, P., and C. Hopkinson. 2003. Ecosystem modulation of dissolved carbon age in a temperate marsh-dominated estuary. Ecosystems 6: 694–705.
Raymond, P.A., J.E. Bauer, and J.J. Cole. 2000. Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnology and Oceanography 45: 1707–1717.
Raymond, P.A., J.E. Bauer, N.F. Caraco, J.J. Cole, B. Longworth, and S.T. Petsch. 2004. Controls on the variability or organic matter and dissolved inorganic carbon ages in northeast US rivers. Marine Chemistry 92: 353–366.
Ribas-Ribas, M., A. Gómez-Parra, and J.M. Forja. 2011. Air–sea CO2 fluxes in the north-eastern shelf of the Gulf of Cádiz (southwest Iberian Peninsula). Marine Chemistry 123: 56–66.
Salisbury, J., D. Vandemark, C. Hunt, J. Campbell, W.R. McGillis, and W. McDowell. 2008. Seasonal observations of surface waters in two Gulf of Maine estuary-plume systems: Relationships between watershed attributes, optical measurements and surface pCO2. Estuarine Coastal and Shelf Science 77(2): 245–252.
Salisbury, J., D. Vandemark, C. Hunt, J. Campbell, B. Jonsson, A. Mahadevan, W. McGillis, and H. Xue. 2009. Episodic riverine influence on surface DIC in the coastal Gulf of Maine. Estuarine Coastal and Shelf Science 82: 108–118.
Sea-bird Electronics. 2013. SBE 43 dissolved oxygen sensor—background information, deployment recommendations, and cleaning and storage. (http://www.seabird.com/application_notes/AN64.htm). Accessed 13 September 2013.
Takahashi, T., S.C. Sutherland, C. Sweeney, A. Poisson, N. Metzl, B. Tilbrook, N. Bates, R. Wanninkhof, R.A. Feely, C. Sabine, J. Olafsson, and Y. Nojiri. 2002. Global air-sea CO2 flux based on climatological surface ocean pCO2 and seasonal biological and temperature effects. Deep-Sea Res 49: 1601–1622.
UNEP-WCMC. 2005. Global Distribution of seagrasses (V2.0, 2005), prepared by the UNEP World Conservation Monitoring Centre (UNEP-WCMC) in collaboration with Dr. Frederick T. Short. (http://data.unep-wcmc.org/datasets/). Accessed 12 June 2013.
Vandemark, D., J.E. Salisbury, C.W. Hunt, S. Shellito, J. Irish, C.L. Sabine, S. Maenner, and W.R. McGillis. 2011. Temporal and spatial dynamics of CO2 air-sea flux in the Gulf of Maine. Journal of Geophysical Research 116, C01012. doi:10.1029/2010JC006408.
Wanninkhof, R., and K. Thoning. 1993. Measurement of fugacity of CO2 in surface water using continuous and discrete sampling methods. Marine Chemistry 44: 189–204.
Zappa, C.J., W.R. McGillis, P.A. Raymond, J.B. Edson, E.J. Hintsa, H.J. Zemmelink, J.W.H. Dacey, and D.T. Ho. 2007. Environmental turbulent mixing controls on air-water gas exchange in marine and aquatic systems. Geophysical Research Letters 34, L10601. doi:10.1029/2006GL028790.
Acknowledgements
This research was supported by NSF Grants 0961825 and 0851447, NASA Carbon—NNX08AL8OG and NOAA Joint Center for Ocean Observation Technology—NA05NOS4731206. We thank the crew of the R/V Gulf Challenger, as well as Kennebec cruise participants including Shawn Shellito, Michael Novak, Christopher Manning, Rebecca Jones, Timothy Moore, and Joshua Brown. Publication funds were provided by the National Aeronautics and Space Agency through the Coastal Carbon Synthesis (CCARS) program(NNX11AD47G).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alberto Vieira Borges
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Hunt, C.W., Salisbury, J.E. & Vandemark, D. CO2 Input Dynamics and Air–Sea Exchange in a Large New England Estuary. Estuaries and Coasts 37, 1078–1091 (2014). https://doi.org/10.1007/s12237-013-9749-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9749-2