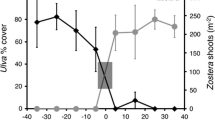
Abstract
Seagrasses are a critical marine habitat and are in decline worldwide. Previous studies have demonstrated that factors such as sediment conditions, resource availability, and desiccation can influence life history transitions and morphology in intertidal eelgrass (Zostera marina L.) and therefore potential for recovery after a disturbance. We combined these factors in an exploratory path model linking environmental conditions to eelgrass vegetative (shoot size and density) and reproductive traits (branching, flowering, seedling recruitment). In this construction, significant path coefficients reveal factors influencing recovery potential. To test the path model, we collected abiotic and eelgrass data at 17 sites in the southern Salish Sea (Washington, USA) and assessed model fit with structural equation modeling. Significant path coefficients linked sediment organic content to shoot size and seedling recruitment, tidal amplitude to reduced flowering, and shoot size and density were inversely correlated. We found no significant links between any morphological or life history trait and nutrient availability, possibly reflecting consistently high nutrients across sites. Variable rates of asexual reproduction and a trade-off between shoot size and density may reflect light limitation in eelgrass’ intertidal range, where light is not expected to be strongly limiting. Overall, structural equation modeling identified organic-rich sediments as relatively more important than desiccation and nutrient conditions for resilience potential of intertidal eelgrass populations in this region. Life history and morphological traits provide eelgrass with recovery mechanisms from disturbance where sediments are muddy, which has implications for both conservation and restoration.


Similar content being viewed by others
References
Backman, T.W.H. 1991. Genotypic and phenotypic variability of Zostera marina on the west coast of North America. Canadian Journal of Botany 69: 1361–1371.
Baden, S.P., and C. Boström. 2001. The leaf canopy of seagrass beds: faunal community structure and function in a salinity gradient along the Swedish coast. In Ecological Comparisons of Sedimentary Shores, ed. K. Reise. Ecological Studies 151: 213–236.
Barko, J.W., and R.M. Smart. 1983. Effects of organic matter additions to sediment on the growth of aquatic plants. Journal of Ecology 71: 161–175.
Berry, H.D., A.T. Sewell, S. Wyllie-Echeverria, B.R. Reeves, T.F. Mumford, Jr., J.R. Skalski, R.C. Zimmerman, and J. Archer. 2003. Puget sound submerged vegetation monitoring project: 2000-2002 monitoring report. Nearshore Habitat Program, Washington State Department of Resources. Olympia, WA. 60 pp. plus appendices.
Boese, B.L., and B.D. Robbins. 2008. Effects of erosion and macroalgae on intertidal eelgrass (Zostera marina) in a northeastern Pacific estuary (USA). Botanica Marina 51: 247–257.
Boese, B.L., J.E. Kaldy, P.J. Clinton, P.M. Eldridge, and C.L. Folger. 2009. Recolonization of intertidal Zostera marina L. (eelgrass) following experimental shoot removal. Journal of Experimental Marine Biology and Ecology 374(1): 69–77.
Burkholder, J.M., D.A. Tomasko, and B.W. Touchette. 2007. Seagrasses and eutrophication. Journal of Experimental Marine Biology and Ecology 350(1–2): 46–72.
Dennison, W.C., and R.S. Alberte. 1985. Role of daily light period in the depth distribution of Zostera marina (eelgrass). Marine Ecology Progress Series 25: 51–61.
Duarte, C.M. 1990. Seagrass nutrient content. Marine Ecology Progress Series 67: 201–207.
Duarte, C.M. 1991. Allometric scaling of seagrass form and productivity. Marine Ecology Progress Series 77: 289–300.
Duffy, J.E. 2006. Biodiversity and functioning of seagrass ecosystems. Marine Ecology Progress Series 311: 233–250.
Ferdie, M., and J.W. Fourqurean. 2004. Responses of seagrass communities to fertilization along a gradient of relative availability of nitrogen and phosphorus in a carbonate environment. Limnology and Oceanography 49: 2082–2094.
Gaeckle, J., P. Dowty, H. Berry, and L. Ferrier. 2011. Puget Sound Submerged Vegetation Monitoring Project 2009 Monitoring Report. Nearshore Habitat Program, Washington State Department of Natural Resources. Olympia, WA. 56 pp. plus appendices.
Grace, J.B. 2006. Structural equation modeling and natural systems. New York: Cambridge University Press.
Gray, J.S., and M. Elliott. 2009. Ecology of marine sediments: from science to management, 2nd ed, 22–27. New York: Oxford University Press.
Greve, T.M., D. Krause-Jensen, M.B. Rasmusson, and P.B. Christensen. 2005. Means of rapid eelgrass (Zostera marina L.) recolonisation in former dieback areas. Aquatic Botany 82: 143–156.
Harrison, P.G. 1993. Variations in demography of Zostera marina and Z. noltii on an intertidal gradient. Aquatic Botany 45: 63–77.
Harwell, M.C., and R.J. Orth. 1999. Eelgrass (Zostera marina L.) seed protection for field experiments and implications for large-scale restoration. Aquatic Botany 64: 51–61.
Hauxwell, J., J. Cebrian, and I. Valiela. 2006. Light dependence of Zostera marina annual growth dynamics in estuaries subject to different degrees of eutrophication. Aquatic Botany 84(1): 17–25.
Hessing-Lewis, M.L., S.D. Hacker, B.A. Menge, and S.S. Rumrill. 2011. Context-dependent eelgrass-macroalgae interactions along an estuarine gradient in the Pacific Northwest, USA. Estuar. Coast. 34: 1169–1181.
Hooper, D., J. Coughlan, and M.R. Mullen. 2008. Structural equation modelling: guidelines for determining model fit. The Electronic Journal of Business Research Methods. 6(1): 53–60.
Hu, L., and P.M. Bentler. 1999. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 6(1): 1–55.
Hughes, A.R., S.L. Williams, C.M. Duarte, K.L. Heck Jr., and M. Waycott. 2009. Associations of concern: declining seagrasses and threatened dependent species. Frontiers in Ecology and the Environment 7: 242–246.
Jarvis, J.C., and K.A. Moore. 2010. The role of seedlings and seed bank viability in the recovery of Chesapeake Bay, USA, Zostera marina populations following a large-scale decline. Hydrobiologia 649: 55–68.
Kaldy, J. 2009. Water column and sediment nutrients as limits to growth of Zostera marina and Thalassia testudinum. In Seagrasses and protective criteria: A review and assessment of research status, ed. W.G. Nelson. Washington, D.C: Office of Research and Development, National Health and Environmental Effects Research Laboratory, EPA/600/R-09/050.
Kentula, M.E., and C.D. McIntire. 1986. The autecology and production dynamics of eelgrass (Zostera marina L) in Netarts Bay, Oregon. Estuaries 9(3): 188–199.
Koch, E.W. 2001. Beyond light: physical, geological, and geochemical parameters as possible submersed aquatic vegetation habitat requirements. Estuaries 24: 1–17.
Koch, E.W., J.D. Ackerman, J. Verduin, and M. van Keulen. 2006. Fluid dynamics in seagrass ecology—from molecules to ecosystems. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth, and C.M. Duarte, 193–225. Dordrecht: Springer.
Marion, S.R., and R.J. Orth. 2012. Seedling establishment in eelgrass: seed burial effects on winter losses of developing seedlings. Marine Ecology Progress Series 448: 197–207.
Marbà, N., and C.M. Duarte. 1998. Rhizome elongation and seagrass clonal growth. Marine Ecology Progress Series 174: 269–280.
Marbà, N., M. Holmer, E. Gacia, and C. Barròn. 2006. Seagrass beds and coastal biogeochemistry. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth, and C.M. Duarte, 135–157. Dordrecht: Springer.
Moore, K.A., R.J. Orth, and J.F. Nowak. 1993. Environmental regulation of seed germination in Zostera marina L. (eelgrass) in Chesapeake Bay: effects of light, oxygen and sediment burial. Aquatic Botany 45: 79–91.
Moore, K.A., and F.T. Short. 2006. Zostera: Biology, ecology, and management. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth, and C.M. Duarte, 361–386. Dordrecht: Springer.
Nixon, S., B. Buckley, S. Granger, and J. Bintz. 2001. Responses of very shallow marine ecosystems to nutrient enrichment. Human Ecological Risk Assessment 7(5): 1457–1481.
Olesen, B., and K. Sand-Jensen. 1994. Biomass-density patterns in the temperate seagrass Zostera marina. Marine Ecology Progress Series 109: 283–291.
Olesen, B. 1999. Reproduction in Danish eelgrass (Zostera marina L.) stands: size-dependence and biomass partitioning. Aquatic Botany 65: 209–219.
Olesen, B., N. Marbà, C.M. Duarte, R.S. Savela, and M.D. Fortes. 2004. Recolonization dynamics in a mixed seagrass meadow: the role of clonal versus sexual processes. Estuaries 27: 770–780.
Orth, R.J., T.J.B. Carruthers, W.C. Dennison, C.M. Duarte, J.W. Fourqurean, K.L. Heck Jr., A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, S. Olyarnik, F.T. Short, M. Waycott, and S.L. Williams. 2006. A global crisis for seagrass ecosystems. BioScience 56: 987–996.
Orth, R.J., K.A. Moore, S.R. Marion, D.J. Wilcox, and D.B. Parrish. 2012. Seed addition facilitates eelgrass recovery in a coastal bay system. Marine Ecology Progress Series 448: 177–195.
Phillips, R.C., W.E. Grant, and C.P. McRoy. 1983. Reproductive strategies of eelgrass (Zostera marina L.). Aquatic Botany 16: 1–20.
Phillips, R.C., and E.G. Meñez. 1988. Seagrasses. Smithsonian contributions to the marine science series, no. 34. Washington, D.C.: Smithsonian Institution Press.
Plus, M., J.M. Deslous-Paoli, and F. Dagault. 2003. Seagrass (Zostera marina L.) bed recolonisation after anoxia-induced full mortality. Aquatic Botany 77: 121–134.
R Development Core Team. 2011. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
Roberts Jr., M.H., R.J. Orth, and K.A. Moore. 1984. Growth of Zostera marina L. seedlings under laboratory conditions of nutrient enrichment. Aquatic Botany 20: 321–328.
Rosseel, Y., D. Oberski, and J. Byrnes. 2011. lavaan: Latent variable analysis. R package version 0.4-10. http://CRAN.R-project.org/package=lavaan.
Ruesink, J.L., J.S. Hong, L. Wisehart, S.D. Hacker, B.R. Dumbauld, M. Hessing-Lewis, and A.C. Trimble. 2010. Congener comparison of native (Zostera marina) and introduced (Z. japonica) eelgrass at multiple scales within a Pacific Northwest estuary. Biological Invasions 12: 1773–1789.
Ruesink, J.L., J.P. Fitzpatrick, B.R. Dumbauld, S.D. Hacker, A.C. Trimble, E.L. Wagner, and L.M. Wisehart. 2012. Life history and morphological shifts in an intertidal seagrass following multiple disturbances. Journal of Experimental Marine Biology and Ecology 424–425: 25–31.
Schanz, A., H. Julich, L. Ferrier, and H. Berry. 2010. Eelgrass stressor-response report 2007–2008: Zostera marina L. (eelgrass) transplant growth and survival along a spatial and tidal gradient in Westcott Bay. Olympia, WA: Washington State Department of Natural Resources.
Short, F.T., and S. Wyllie-Echeverria. 1996. Natural and human-induced disturbance of seagrasses. Environmental Conservation 23: 17–27.
Specht, D.T., and B.L. Boese. 2009. The effects of temperature and desiccation on the seagrasses Zostera marina and Thalassia testudinum. In Seagrasses and protective criteria: A review and assessment of research status, ed. W.G. Nelson. Newport, OR: Western Ecology Division, National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, EPA/600/R-09/050.
Thom, R.M., H.L. Diefenderfer, J. Vavrinec, and A.B. Borde. 2012. Restoring resiliency: case studies from Pacific Northwest estuarine eelgrass (Zostera marina L.) ecosystems. Estuaries and Coasts 35: 78–91.
Valdemarsen, T., P. Canal-Vergés, E. Kristensen, M. Holmer, M.D. Kristiansen, and M.R. Flindt. 2010. Vulnerability of Zostera marina seedlings to physical stress. Marine Ecology Progress Series 418: 119–130.
van Katwijk, M.M., and L.J.M. Wijgergangs. 2004. Effects of locally varying exposure, sediment type and low-tide water cover on Zostera marina recruitment from seed. Aquatic Botany 80: 1–12.
Vermaat, J.E. 2009. Linking clonal growth patterns and ecophysiology allows the prediction of meadow-scale dynamics of seagrass beds. Perspectives in Plant Ecology 11: 137–155.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnike, A. Calladine, J.W. Fourqurean, K.L. Heck Jr., A.R. Hughes, G.A. Kendrick, W.J. Kenworthy, F.T. Short, and S.L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences 106: 12377–12381.
Wicks, E.C., E.W. Koch, J.M. O’Neil, and K. Elliston. 2009. Effects of sediment organic content and hydrodynamic conditions on the growth and distribution of Zostera marina. Marine Ecology Progress Series 378: 71–80.
Wisehart, L.M., B.R. Dumbauld, J.L. Ruesink, and S.D. Hacker. 2007. Importance of eelgrass early life history stages in response to oyster aquaculture disturbance. Marine Ecology Progress Series 344: 71–80.
Acknowledgments
We sincerely thank everyone who made this huge field effort possible: Amy Glaub, Michael Hannam, Kevin See, Lauren Sullenberger, Ginger Tennant, and students in the Spring 2007 Marine Ecology course at University of Washington: Demian Bailey, Sean Cappello, Matt Chin, Anna Choi, Nhuchi Dao, Heather Deboli, Greg Dunster, Anna Grishchenko, Ken Hamel, Tenecia Harris, Michael Klaczynski, Kelsea Laegreid, Justin Neste, Natalee Nusslock, Cody Nutsch, Stephanie Raghubeer, Jennifer Reitz, Elizabeth Suguira, Chaochung Tsai, and Pamela Williams. We appreciate the guidance we received from Helen Berry, Pete Dowty, Jeffrey Gaeckle, Thomas Mumford, Anja Schanz, Ron Thom, Sandy Wyllie-Echeverria, Laura Feinstein, and the insightful editorial comments provided by Janneke HilleRisLambers, and two anonymous reviewers. Thanks also to the following for access to private tidelands: Paul Blau (Blau Oyster Company), Doug Bulthuis (Padilla Bay National Estuarine Research Reserve), Betty Carteret, Bill Dewey (Taylor Shellfish Farms), Harry Eiesland, John and Brit Glomset, Mike MacKay (Lummi Tribe), and Eric Shen. This study was supported by funds from the Environmental Protection Agency Science to Achieve Results, Achievement Rewards for College Scientists fellowships, and National Science Foundation GK-12 Ocean and Coastal Interdisciplinary Science Program Fellowship (to S. Yang), Washington State Department of Natural Resources and Department of Ecology (to E. Wheat), and the Western Regional Aquaculture Center through grant no. 2004-38500-14698 from the United States Department of Agriculture, Cooperative State Research, Education, and Extension Service (to J. Ruesink).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 99 kb)
Appendix: Methods to Cull Correlated Metrics of Desiccation Stress and Sediment Conditions
Appendix: Methods to Cull Correlated Metrics of Desiccation Stress and Sediment Conditions
We explored the following metrics relevant to desiccation stress before settling on tidal amplitude and elevation: (1) tidal elevation, (2) estimated hours inundated in the previous year (2006) using tidal elevation and predicted water levels by hour (http://tbone.biol.sc.edu/tide/), (3) relative tidal amplitude (the difference between mean lower low water and mean higher high water on May 18, 2007), and (4) relative time of day at low tide during summer using tidal predictions (as a proxy for air temperature). Tidal elevation and inundation time were correlated (r(29) = −0.95, p < 0.0001), and tidal amplitude and time at low tide were correlated (r(32) = 0.34, p = 0.046).
We explored the following metrics of sediment conditions before settling on sediment organic content: (1) beach slope, in two categories: extensive broad shallows in embayments or pocket beaches (“flats”) versus narrow, sloping shorelines (“fringes”) (Berry et al. 2003), (2) silt to sand ratio, and (3) sediment organic matter. Categorical beach slope (r(29) = −0.53, p = 0.002) and silt to sand ratio (r(29) = 0.85, p < < 0.0001) were highly correlated with sediment organic matter. Sediment organic content is controlled by biological processes, including heterotrophic assimilation and autochthonous production, as well as physical processes. Thus, we used it to indicate relative differences in biologically relevant sediment conditions among our sites.
Rights and permissions
About this article
Cite this article
Yang, S., Wheat, E.E., Horwith, M.J. et al. Relative Impacts of Natural Stressors on Life History Traits Underlying Resilience of Intertidal Eelgrass (Zostera marina L.). Estuaries and Coasts 36, 1006–1013 (2013). https://doi.org/10.1007/s12237-013-9609-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9609-0