Abstract
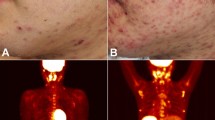
Chronic active Epstein–Barr virus (EBV) disease (CAEBV) is a systemic EBV-positive lymphoproliferative disorder characterized by fever, lymphadenopathy, and splenomegaly. Patients with CAEBV may present with cutaneous symptoms, including hypersensitivity to mosquito bites and hydroa vacciniforme (HV)-like eruptions. HV is a rare photodermatosis characterized by vesicles and crust formation after exposure to sunlight, with onset in childhood, and is associated with latent EBV infection. While γδ T cells have recently been demonstrated to be the major EBV-infected cell population in HV, the immunophenotypic features of EBV-infected γδ T cells in CAEBV with HV-like eruptions or HV remain largely undetermined. We describe three patients with CAEBV whose γδ T cells were found to be the major cellular target of EBV. HV-like eruptions were observed in two of these patients. A clonally expanded subpopulation of γδ T cells that were highly activated and T cell receptor Vγ9- and Vδ2-positive cells was demonstrated in all patients. We also show that the clonally expanded γδ T cells infiltrated into the HV-like eruptions in one patient from whom skin biopsy specimens were available. These results suggest the pathogenic roles of clonally expanded γδ T cells infected by EBV in patients with CAEBV and HV-like eruptions.




Similar content being viewed by others
References
Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu Rev Immunol. 2007;25:587–617.
Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein–Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. 2009;20:1472–82.
Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S, Morio T, Mori M, Yokota S, Imashuku S. Proposed guidelines for diagnosing chronic active Epstein–Barr virus infection. Am J Hematol. 2005;80:64–9.
Kimura H, Hoshino Y, Hara S, Sugaya N, Kawada J, Shibata Y, Kojima S, Nagasaka T, Kuzushima K, Morishima T. Differences between T cell-type and natural killer cell-type chronic active Epstein–Barr virus infection. J Infect Dis. 2005;191:531–9.
Gupta G, Man I, Kemmett D. Hydroa vacciniforme: a clinical and follow-up study of 17 cases. J Am Acad Dermatol. 2000;42:208–13.
Chen HH, Hsiao CH, Chiu HC. Hydroa vacciniforme-like primary cutaneous CD8-positive T-cell lymphoma. Br J Dermatol. 2002;147:587–91.
Iwatsuki K, Xu Z, Takata M, Iguchi M, Ohtsuka M, Akiba H, Mitsuhashi Y, Takenoshita H, Sugiuchi R, Tagami H, Kaneko F. The association of latent Epstein–Barr virus infection with hydroa vacciniforme. Br J Dermatol. 1999;140:715–21.
Cho KH, Lee SH, Kim CW, Jeon YK, Kwon IH, Cho YJ, Lee SK, Suh DH, Chung JH, Yoon TY, Lee SJ. Epstein–Barr virus-associated lymphoproliferative lesions presenting as a hydroa vacciniforme-like eruption: an analysis of six cases. Br J Dermatol. 2004;151:372–80.
Iwatsuki K, Satoh M, Yamamoto T, Oono T, Morizane S, Ohtsuka M, Xu ZG, Suzuki D, Tsuji K. Pathogenic link between hydroa vacciniforme and Epstein–Barr virus-associated hematologic disorders. Arch Dermatol. 2006;142:587–95.
Kimura H, Miyake K, Yamauchi Y, Nishiyama K, Iwata S, Iwatsuki K, Gotoh K, Kojima S, Ito Y, Nishiyama Y. Identification of Epstein–Barr virus (EBV)-infected lymphocyte subtypes by flow cytometric in situ hybridization in EBV-associated lymphoproliferative diseases. J Infect Dis. 2009;200:1078–87.
Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, Kawa K, Ohshima K, Nakamura S. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–86.
Hirai Y, Yamamoto T, Kimura H, Ito Y, Tsuji K, Miyake T, Morizane S, Suzuki D, Fujii K, Iwatsuki K. Hydroa vacciniforme is associated with increased numbers of Epstein–Barr virus-infected γδ T Cells. J Invest Dermatol. 2012;132:1401–8.
Tanaka C, Hasegawa M, Fujimoto M, Iwatsuki K, Yamamoto T, Yamada K, Kawa K, Saikawa Y, Toga A, Mase S, Wada T, Takehara K, Yachie A. Phenotypic analysis in a case of hydroa vacciniforme-like eruptions associated with chronic active Epstein–Barr virus disease of γδ T cells. Br J Dermatol. 2012;166:216–8.
Toga A, Wada T, Sakakibara Y, Mase S, Araki R, Tone Y, Toma T, Kurokawa T, Yanagisawa R, Tamura K, Nishida N, Taneichi H, Kanegane H, Yachie A. Clinical significance of cloned expansion and CD5 down-regulation in Epstein–Barr virus (EBV)-infected CD8+ T lymphocytes in EBV-associated hemophagocytic lymphohistiocytosis. J Infect Dis. 2010;201:1923–32.
de Villartay JP, Lim A, Al-Mousa H, Dupont S, Dechanet-Merville J, Coumau-Gatbois E, Gougeon ML, Lemainque A, Eidenschenk C, Jouanguy E, Abel L, Casanova JL, Fischer A, Le Deist F. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115:3291–9.
Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kuhr J, Mascart F, Schmitt-Graeff A, Niemeyer C, Fisch P. A variant of SCID with specific immune responses and predominance of γδ T cells. J Clin Invest. 2005;115:3140–8.
Wada T, Schurman SH, Otsu M, Garabedian EK, Ochs HD, Nelson DL, Candotti F. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc Natl Acad Sci USA. 2001;98:8697–702.
van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317.
Quintanilla-Martinez L KH, Jaffe ES. EBV-positive T-cell lymphoproliferative disorders of childhood. Geneva, Switzerland: World Health Organization, 2008.
Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49.
O’Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. γδ T-cell receptors: functional correlations. Immunol Rev. 2007;215:77–88.
Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994;153:3979–88.
Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612.
Oyoshi MK, Nagata H, Kimura N, Zhang Y, Demachi A, Hara T, Kanegane H, Matsuo Y, Yamaguchi T, Morio T, Hirano A, Shimizu N, Yamamoto K. Preferential expansion of Vγ9-JγP/Vδ2-Jδ3 γδ T cells in nasal T-cell lymphoma and chronic active Epstein–Barr virus infection. Am J Pathol. 2003;162:1629–38.
Komori HK, Meehan TF, Havran WL. Epithelial and mucosal gamma delta T cells. Curr Opin Immunol. 2006;18:534–8.
Verneuil L, Gouarin S, Comoz F, Agbalika F, Creveuil C, Varna M, Vabret A, Janin A, Leroy D. Epstein–Barr virus involvement in the pathogenesis of hydroa vacciniforme: an assessment of seven adult patients with long-term follow-up. Br J Dermatol. 2010;163:174–82.
Yoshimasu T, Nishide T, Seo N, Hiroi A, Ohtani T, Uede K, Furukawa F. Susceptibility of T cell receptor-alpha chain knock-out mice to ultraviolet B light and fluorouracil: a novel model for drug-induced cutaneous lupus erythematosus. Clin Exp Immunol. 2004;136:245–54.
Acknowledgments
We thank Ms Harumi Matsukawa and Ms. Shizu Kouraba for their excellent technical assistance. This work was supported by a grant from Takeda Science Foundation, Osaka; a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the Ministry of Health, Labour, and Welfare of Japan, Tokyo.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wada, T., Toga, A., Sakakibara, Y. et al. Clonal expansion of Epstein–Barr virus (EBV)-infected γδ T cells in patients with chronic active EBV disease and hydroa vacciniforme-like eruptions. Int J Hematol 96, 443–449 (2012). https://doi.org/10.1007/s12185-012-1156-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1156-0