Abstract
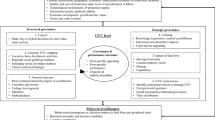
Until recently, consensus existed in certain circles that the African industry was not suitable for cost-effective production of quality, safe drugs. Yet, public and private pharmaceutical enterprises have cropped up on the continent, with some venturing into production of sophisticated and complex drugs, such as antiretrovirals (ARVs). In our study, we analyse and contrast the dynamics of local pharmaceutical manufacturing in Mozambique and Zimbabwe with the objective of understanding why pharmaceutical production in Africa is picking up momentum and the influence of global funding for ARVs in this process. Our analysis identifies two routes of development for local pharmaceutical manufacturing: a favourable economic outlook and support from the international community created the necessary conditions for the development of the nascent pharmaceutical industry in Mozambique, while in Zimbabwe, the presence of an established local industry was instrumental in bringing in favourable, if not always coherent, government regulation. In both countries, the introduction of AIDS treatment created windows of opportunity for local production of pharmaceuticals by increasing public sector demand, providing fresh funds, and providing a justification for government regulation favouring local production. Despite the long-standing and well-known problems that created persistent shortcomings in human resources and in the economic and industrial environments, we conclude that pre-existing developmental roots, international funds and supportive state industrial policies are encouraging more manufacturers to enter the business of local pharmaceutical production in Africa. However, the opportunities brought in by fresh AIDS funds will need to be sensibly managed at both the local and global levels, as the world’s interest on the disease may not last in the long term.

Similar content being viewed by others
Notes
Such as Saidal in Algeria and Saphad in Tunisia.
For example, CIPLA and Quality Pharmaceuticals in Uganda.
General notice 240 of 2002; ‘2. In view of the rapid spread of HIV/AIDS among the population of Zimbabwe, the Minister hereby declares an emergency for a period of 6 months, with effect from the date of promulgation of this notice, for the purpose of enabling the state or a person authorised by the Minister under section 34 of the Act (a) to make or use any patented drug, including any antiretroviral drug, used in the treatment of persons suffering from HIV/AIDS or HIV/AIDS-related conditions and (b) to import any generic drug used in the treatment of persons suffering from HIV/AIDS or HIV/AIDS-related conditions’. Source: http://www.wipo.int/wipolex/en/text.jsp?file_id=214688 accessed 25 February 2014
References
Banda G. Finance as a ‘forgotten technological capability’ for promoting African local pharmaceutical manufacture. Int J Technol Manag Sustain Dev. 2013;12(2):117–35. doi:10.1386/tmsd.12.2.117_1.
Banda G. Financing ARV drug manufacture in Zimbabwe: implications for technological capability upgrading and innovation for African local pharmaceutical production. PhD Thesis. UK: The Open University 2012
Barker C. The Mozambique pharmaceutical policy. Lancet. 1983;2(8353):780–82.
Beall R, Kuhn R. Trends in compulsory licensing of pharmaceuticals since the Doha declaration: a database analysis. PLoS Med. 2012;9(1):e1001154. doi:10.1371/journal.pmed.1001154.
Benatar SR. Health care reform and the crisis of HIV and AIDS in South Africa. N Engl J Med. 2004;351(1):81–92. doi:10.1056/NEJMhpr033471.
Brett EA. From corporatism to liberalization in Zimbabwe: economic policy regimes and political crisis, 1980–97. Int Polit Sci Rev. 2005;26(1):91–106. doi:10.1177/0192512105047898.
Cameron A, Ewen M, Rossdegnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240–49. doi:10.1016/S0140-6736(08)61762-6.
Chaudhuri S, Mackintosh M, Mujinja PGM. Indian generics producers, access to essential medicines and local production in Africa: an argument with reference to Tanzania. Eur J Dev Res. 2010;22(4):451–68. doi:10.1057/ejdr.2010.27.
CMAM. “Proposta de Necessidades E Alocação de Recursos Para Medicamentos”. Ministério da Saúde de Moçambique 2011.
COWI. Finalização do plano de negócios da SMM: situação dos mercados farmacêuticos Em Moçambique E Na Região Da SADC. Maputo: COWI Consulting, Africa; 2012.
De Oliveira L. Inicitativa de instalação Da fábrica de antiretrovirrais E outros medicamentos Em moçambique; avaliação Do projecto”. Fundação Oswaldo Cruz: Farmanguinhos; 2013.
Fairbairn M. Indirect dispossession: domestic power imbalances and foreign access to land in Mozambique. Dev Chang. 2013;44(2):335–56. doi:10.1111/dech.12013.
Flynn M. Public production of anti-retroviral medicines in Brazil, 1990–2007. Dev Chang. 2008;39(4):513–36. doi:10.1111/j.1467-7660.2008.00494.x.
Froese EH. Meeting the pharmaceutical needs of a developing country. World Health Forum. 1991;12(1):25–8.
GIZ. Bringing Medicines to Low-Income Markets—A Guide to Creating Inclusive Business Models for Pharmaceutical Companies. German Federal Ministry for Economic Cooperation and Development (BMZ). 2012. http://www2.gtz.de/dokumente/bib-2012/giz2012-0025en-medicines-low-income-markets.pdf.
GoM. Diploma ministerial No52/2010 Sobre Fixação de Preços de Medicamentos. 2010.
Institute for Health Metrics and Evaluation, and Human Development Network the World Bank. The Global Burden of Disease: Generating Evidence, Guiding Policy Sub-Saharan Africa Regional Edition. The University of Washington and the Human Development Network. Washington, DC: World Bank. 2013. http://documents.worldbank.org/curated/en/2013/08/18187588/global-burden-disease-generating-evidence-guiding-policy-sub-saharan-africa-regional-edition.
Kaplan W, Laing R. Local production of pharmaceuticals: industrial policy and access to medicines: an overview of key concepts, issues and opportunities for future research. 32036. HPN Discussion Paper. The World Bank. 2005. http://www.who.int/medicines/technical_briefing/tbs/KaplanLocalProductionFinal5b15d.pdf.
Kuanpoth J. Patents and access to antiretroviral medicines in Vietnam after world trade organization accession. J World Intellect Prop. 2007;10(3–4):201–24. doi:10.1111/j.1747-1796.2007.00321.x.
MISAU. “Plano Estratégico Da Área Farmacêutica”. Departamento Farmacêutico do Ministério da Saúde da República de Moçambique. 1996.
Mujinja PGM, Mackintosh M, Justin-Temu M, Wuyts M. Local production of pharmaceuticals in Africa and access to essential medicines: ‘urban bias’ in access to imported medicines in Tanzania and its policy implications. Glob Health. 2014;10(1):12. doi:10.1186/1744-8603-10-12.
Nicol D, Owoeye O. Using TRIPS flexibilities to facilitate access to medicines. Bull World Health Organ. 2013;91(7):533–39. doi:10.2471/BLT.12.115865.
Orsi F, D’Almeida C, Lia H, Mamadou C, Paulo T, Benjamin C. TRIPS post-2005 and access to new antiretroviral treatments in southern countries: issues and challenges. AIDS. 2007;21(15):1997–2003. doi:10.1097/QAD.0b013e328273bbe4.
Osewe PL, Nkrumah YK, Sackey EK. Improving access to HIV/AIDS medicines in Africa: Trade-Related Aspects of Intellectual Property Rights (TRIPS) flexibilities utilization. World Bank Publications. 2008.
Owoeye OA. Compulsory patent licensing and local drug manufacturing capacity in Africa. Bull World Health Organ. 2014;92(3):214–19. doi:10.2471/BLT.13.128413.
Pavignani E, Durão JR. Managing external resources in Mozambique: building new aid relationships on shifting sands? Health Policy Plan. 1999;14(3):243–53.
Pfeiffer J, Montoya P, Alberto J, Baptista MK, de Morais M, Pugas MM, et al. Integration of HIV/AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique—a case study. J Int AIDS Soc. 2010;13(1):3. doi:10.1186/1758-2652-13-3.
Pinheiro E, Vasan A, Kim JY, Lee E, Guimier JM, Perriens J. Examining the production costs of antiretroviral drugs. AIDS (London, England). 2006;20(13):1745–52. doi:10.1097/01.aids.0000242821.67001.65.
Portal V. Lula Visita 1a Fábrica de Remédios Contra Aids Em Moçambique—Portal Vermelho. 2010. http://www.vermelho.org.br/noticia/141228-9.
Rovira J. Creating and promoting domestic drug manufacturing capacities: a solution for developing countries? In: In Negot heal intellect prop access mediciens. Sterling: International Centre for Trade and Sustainable Development; 2006.
Russo G, McPake B. Medicine prices in urban Mozambique: a public health and economic study of pharmaceutical markets and price determinants in low-income settings. Health Policy Plan. 2010;25(1):70–84. doi:10.1093/heapol/czp042.
Russo G, Cabral L, Ferrinho P. Brazil-Africa technical cooperation in health: what’s its relevance to the post-Busan debate on ‘aid effectiveness’? Glob Health. 2013;9:2. doi:10.1186/1744-8603-9-2.
Russo G, de Oliveira L, Shankland A, Sitoe T. On the margins of aid orthodoxy: the Brazil-Mozambique collaboration to produce essential medicines in Africa. Glob Health. 2014;10(1):70. doi:10.1186/s12992-014-0070-z.
Sacco S. 2004. A comparative study of the implementation in ZIMBABWE and south africa of the international law rules that allow compulsory licensing and parallel importation for HIV/AIDS drugs. University Phd Dissertation. The American University of Cairo. http://repository.up.ac.za/xmlui/bitstream/handle/2263/1100/sacco_sf_1.pdf?sequence=1.
Seiter A. A practical approach to pharmaceutical policy. Vol. 55203. Directions in development. Washington: The World Bank, 2010. https://openknowledge.worldbank.org/bitstream/handle/10986/2468/552030PUB0Phar10Box349442B01PUBLIC1.pdf?sequence=4.
Shadlen KC, Fonseca E. Health policy as industrial policy: Brazil in comparative perspective. Polit Soc. 2013;41(4):561–87.
SMM, and Farmanguinhos. Plano de negócios Da SMM. Matola: Sociedade Moçambicana de Medicamentos; 2013.
Turshen M. Reprivatizing pharmaceutical supplies in Africa. J Public Health Policy. 2001;22(2):198–225.
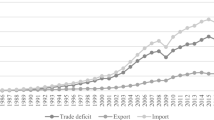
UNCTAD. UNCTADSTAT—Trade Statistical Database. United Nations Conference for Trade and Developtment. 2013. http://unctadstat.unctad.org/ReportFolders/reportFolders.aspx?sCS_referer=&sCS_ChosenLang=en.
UNIDO. Pharmaceutical sector profile: Uganda. Global UNIDO project: strengthening the local production of essential generic drugs in least developed and developing countries. Vienna: United Nations Industrial Development Organization; 2010.
UNIDO. Pharmaceutical sector profile: Zimbabwe. Global UNIDO project: strengthening the local production of essential generic drugs in least developed and developing countries. Vienna: United Nations Industrial Development Organization; 2011.
UNIDO-AUC. Pharmaceutical manufacturing plan for Africa—business plan. Prepared as part of the African union commission-UNIDO partnership. Addis Ababa: United Nations Industrial development Organization; 2012.
Van de Maele N, Evans DB, Tan-Torres T. Development assistance for health in africa: are we telling the right story? Bull World Health Organ. 2013;91(7):483–90. doi:10.2471/BLT.12.115410.
Waning B, Diedrichsen E, Moon S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc. 2010;13:35. doi:10.1186/1758-2652-13-35.
WHO. Local production for access to medical products: developing a framework to improve public health. WHO Public Health Innovation and Intellectual Property. ISBN: 978 92 4 150289 4. 2011. http://www.who.int/phi/publications/local_production_policy_framework/en/.
Wilson KR, Kohler JC, Ovtcharenko N. The make or buy debate: considering the limitations of domestic production in Tanzania. Glob Health. 2012;8(June):20. doi:10.1186/1744-8603-8-20.
World Bank. The World Bank Statistical Database. 2013. http://data.worldbank.org/.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Rights and permissions
About this article
Cite this article
Russo, G., Banda, G. Re-Thinking Pharmaceutical Production in Africa; Insights from the Analysis of the Local Manufacturing Dynamics in Mozambique and Zimbabwe. St Comp Int Dev 50, 258–281 (2015). https://doi.org/10.1007/s12116-015-9186-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12116-015-9186-2