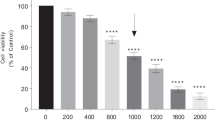
Abstract
Parkinson's disease is a progressive neurodegenerative disease causing tremor, rigidity, bradykinesia, and gait impairment. Oxidative stress and mitochondrial dysfunction play important roles in the development of Parkinson disease. Salidroside (Sal), a phenylpropanoid glycoside isolated from Rhodiola rosea L., has potent antioxidant properties. Previous work from our group suggests that Sal might protect dopaminergic neurons through inhibition of reactive oxygen species (ROS) and nitric oxide (NO) generation. In the present study, we investigated the protective effects of Sal in MPTP/MPP+ models of Parkinson's disease in an attempt to elucidate the underlying mechanism of protection. We found that Sal pretreatment protected dopaminergic neurons against MPTP/MPP+-induced toxicity in a dose-dependent manner by: (1) reducing the production of ROS–NO, (2) regulating the ratio of Bcl-2/Bax, (3) decreasing cytochrome-c and Smac release, and inhibiting caspase-3, caspas-6, and caspas-9 activation, and (4) reducing α-synuclein aggregation. The present study supports the hypothesis that Sal may act as an effective neuroprotective agent through modulation of the ROS–NO-related mitochondrial pathway in vitro and in vivo.









Similar content being viewed by others
Reference
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson's disease. Lancet Neurol 5(6):525–535
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125(Pt 4):861–870
Meissner W, Hill MP, Tison F, Gross CE, Bezard E (2004) Neuroprotective strategies for Parkinson's disease: conceptual limits of animal models and clinical trials. Trends Pharmacol Sci 25(5):249–253
Foltynie T, Kahan J (2013) Parkinson's disease: an update on pathogenesis and treatment. J Neurol 260(5):1433–1440
Shukla V, Mishra SK, Pant HC (2011) Oxidative stress in neurodegeneration. Adv Pharmacol Sci 2011:572634
Martinez TN, Greenamyre JT (2012) Toxin models of mitochondrial dysfunction in Parkinson's disease. Antioxid Redox Signal 16(9):920–934
Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, Mungenast AE, Muffat J, Mitalipova M, Pluth MD, Jui NT, Schule B, Lippard SJ, Tsai LH, Krainc D, Buchwald SL, Jaenisch R, Lindquist S (2013) Identification and rescue of alpha-synuclein toxicity in Parkinson patient-derived neurons. Science 342(6161):983–987
Chaturvedi RK, Beal MF (2013) Mitochondria targeted therapeutic approaches in Parkinson's and Huntington's diseases. Mol Cell Neurosci 55:101–114
Beal MF (2009) Therapeutic approaches to mitochondrial dysfunction in Parkinson's disease. Parkinsonism Relat Disord 15(Suppl 3):S189–S194
Guo S, Bezard E, Zhao B (2005) Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radic Biol Med 39(5):682–695
Sheng QS, Wang ZJ, Zhang J, Zhang YG (2013) Salidroside promotes peripheral nerve regeneration following crush injury to the sciatic nerve in rats. Neuroreport 24(5):217–223
Yin D, Yao W, Chen S, Hu R, Gao X (2009) Salidroside, the main active compound of Rhodiola plants, inhibits high glucose-induced mesangial cell proliferation. Planta Med 75(11):1191–1195
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z (2007) Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564(1–3):18–25
Chen X, Liu J, Gu X, Ding F (2008) Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats. Brain Res 1238:189–198
Cao LL, Du GH, Wang MW (2006) The effect of salidroside on cell damage induced by glutamate and intracellular free calcium in PC12 cells. J Asian Nat Prod Res 8(1–2):159–165
Li X, Ye X, Li X, Sun X, Liang Q, Tao L, Kang X, Chen J (2011) Salidroside protects against MPP(+)-induced apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res 1382:9–18
Tatton WG, Chalmers-Redman RM, Ju WJ, Mammen M, Carlile GW, Pong AW, Tatton NA (2002) Propargylamines induce antiapoptotic new protein synthesis in serum- and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther 301(2):753–764
Ormerod MG, Collins MK, Rodriguez-Tarduchy G, Robertson D (1992) Apoptosis in interleukin-3-dependent haemopoietic cells. Quantification by two flow cytometric methods. J Immunol Methods 153(1–2):57–65
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65(10):1575–1582
Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T (1999) Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38(21):3209–3212
Matsuura K, Kabuto H, Makino H, Ogawa N (1997) Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods 73(1):45–48
Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M (2007) Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 52(8):1678–1684
Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S (1995) Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4(3):257–269
Sriram K, Pai KS, Boyd MR, Ravindranath V (1997) Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res 749(1):44–52
Grunblatt E, Mandel S, Youdim MB (2000) MPTP and 6-hydroxydopamine-induced neurodegeneration as models for Parkinson's disease: neuroprotective strategies. J Neurol 247(Suppl 2):I95–I102
Przedborski S, Vila M (2003) The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson's disease. Ann N Y Acad Sci 991:189–198
Przedborski S, Jackson-Lewis V, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G (2000) The parkinsonian toxin MPTP: action and mechanism. Restor Neurol Neurosci 16(2):135–142
Marsden CD (1990) Parkinson's disease. Lancet 335(8695):948–952
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20(4):415–455
Riederer P, Wuketich S (1976) Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm 38(3–4):277–301
Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE (2001) Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci 21(17):6853–6861
Kasprzak KS (2002) Oxidative DNA and protein damage in metal-induced toxicity and carcinogenesis. Free Radic Biol Med 32(10):958–967
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Stadtman ER (1992) Protein oxidation and aging. Science 257(5074):1220–1224
Maguire-Zeiss KA, Short DW, Federoff HJ (2005) Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson's disease? Brain Res Mol Brain Res 134(1):18–23
Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem 52(2):381–389
Brieger K, Schiavone S, Miller FJ, Krause KH (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659
Patten DA, Germain M, Kelly MA, Slack RS (2010) Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis 20(Suppl 2):S357–S367
Di Monte D, Sandy MS, Ekstrom G, Smith MT (1986) Comparative studies on the mechanisms of paraquat and 1-methyl-4-phenylpyridine (MPP+) cytotoxicity. Biochem Biophys Res Commun 137(1):303–309
Kern JC, Kehrer JP (2005) Free radicals and apoptosis: relationships with glutathione, thioredoxin, and the BCL family of proteins. Front Biosci 10:1727–1738
Cassarino DS, Parks JK, Parker WJ, Bennett JJ (1999) The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta 1453(1):49–62
Calabrese V, Cornelius C, Rizzarelli E, Owen JB, Dinkova-Kostova AT, Butterfield DA (2009) Nitric oxide in cell survival: a Janus molecule. Antioxid Redox Signal 11(11):2717–2739
Del-Bel E, Padovan-Neto FE, Raisman-Vozari R, Lazzarini M (2011) Role of nitric oxide in motor control: implications for Parkinson's disease pathophysiology and treatment. Curr Pharm Des 17(5):471–488
Dawson VL, Dawson TM (1998) Nitric oxide in neurodegeneration. Prog Brain Res 118:215–229
Zhang L, Dawson VL, Dawson TM (2006) Role of nitric oxide in Parkinson's disease. Pharmacol Ther 109(1–2):33–41
Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM (1996) Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A 93(10):4565–4571
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5(12):1403–1409
Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM (1999) Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A 96(10):5774–5779
Schmitt K, Grimm A, Kazmierczak A, Strosznajder JB, Gotz J, Eckert A (2012) Insights into mitochondrial dysfunction: aging, amyloid-beta, and tau-A deleterious trio. Antioxid Redox Signal 16(12):1456–1466
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87(1):99–163
Schapira AH (2006) Mitochondrial disease. Lancet 368(9529):70–82
Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183
Du L, Mei HF, Yin X, Xing YQ (2014) Delayed growth of glioma by a polysaccharide from Aster tataricus involve upregulation of Bax/Bcl-2 ratio, activation of caspase-3/8/9, and downregulation of the Akt. Tumour Biol 35:1819–1825
Graham RK, Ehrnhoefer DE, Hayden MR (2011) Caspase-6 and neurodegeneration. Trends Neurosci 34(12):646–656
Chu Y, Mickiewicz AL, Kordower JH (2011) alpha-Synuclein aggregation reduces nigral myocyte enhancer factor-2D in idiopathic and experimental Parkinson's disease. Neurobiol Dis 41(1):71–82
Adamczyk A, Kazmierczak A, Strosznajder JB (2006) alpha-Synuclein and its neurotoxic fragment inhibit dopamine uptake into rat striatal synaptosomes. Relationship to nitric oxide. Neurochem Int 49(4):407–412
Tsang AH, Chung KK (2009) Oxidative and nitrosative stress in Parkinson's disease. Biochim Biophys Acta 1792(7):643–650
Chu Y, Mickiewicz AL, Kordower JH (2011) alpha-Synuclein aggregation reduces nigral myocyte enhancer factor-2D in idiopathic and experimental Parkinson's disease. Neurobiol Dis 41(1):71–82
Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2009) alpha-Synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol 41(10):2015–2024
Singh S, Dikshit M (2007) Apoptotic neuronal death in Parkinson's disease: involvement of nitric oxide. Brain Res Rev 54(2):233–250
Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P (2004) alpha-Synuclein and Parkinson's disease. FASEB J 18(6):617–626
Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S, Souza JM, Jackson-Lewis V, Lee VM, Ischiropoulos H (2001) Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. J Neurochem 76(2):637–640
Maguire-Zeiss KA (2008) alpha-Synuclein: a therapeutic target for Parkinson's disease? Pharmacol Res 58(5–6):271–280
Acknowledgment
This work was supported by grants from the Nature Science Foundation of China (Project No. 81173590).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The authors Songhai Wang and Hong He contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, S., He, H., Chen, L. et al. Protective Effects of Salidroside in the MPTP/MPP+-Induced Model of Parkinson's Disease through ROS–NO-Related Mitochondrion Pathway. Mol Neurobiol 51, 718–728 (2015). https://doi.org/10.1007/s12035-014-8755-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8755-0