Abstract
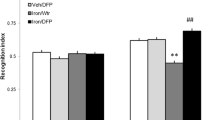
We have recently shown that chronic treatment with cannabidiol (CBD) was able to recover memory deficits induced by brain iron loading in a dose-dependent manner in rats. Brain iron accumulation is implicated in the pathogenesis of neurodegenerative diseases, including Parkinson’s and Alzheimer’s, and has been related to cognitive deficits in animals and human subjects. Deficits in synaptic energy supply have been linked to neurodegenerative diseases, evidencing the key role played by mitochondria in maintaining viable neural cells and functional circuits. It has also been shown that brains of patients suffering from neurodegenerative diseases have increased expression of apoptosisrelated proteins and specific DNA fragmentation. Here, we have analyzed the expression level of brain proteins involved with mitochondrial fusion and fission mechanisms (DNM1L and OPA1), the main integral transmembrane protein of synaptic vesicles (synaptophysin), and caspase 3, an apoptosis-related protein, to gain a better understanding of the potential of CBD in restoring the damage caused by iron loading in rats. We found that CBD rescued iron-induced effects, bringing hippocampal DNM1L, caspase 3, and synaptophysin levels back to values comparable to the control group. Our results suggest that iron affects mitochondrial dynamics, possibly trigging synaptic loss and apoptotic cell death and indicate that CBD should be considered as a potential molecule with memory-rescuing and neuroprotective properties to be used in the treatment of cognitive deficits observed in neurodegenerative disorders.








Similar content being viewed by others
References
Stankiewicz JM, Brass SD (2009) Role of iron in neurotoxicity: a cause for concern in the elderly? Curr Opin Clin Nutr Metab Care 12(1):22–29
Mills E, Dong XP, Wang F, Xu H (2010) Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med Chem 2(1):51–64
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE et al (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114(Pt 4):1953–1975
Hirsch EC, Brandel JP, Galle P, Javoy-Agid F, Agid Y (1991) Iron and aluminum increase in the substantia nigra of patients with Parkinson's disease: an X-ray microanalysis. J Neurochem 56(2):446–451
Good PF, Perl DP, Bierer LM, Schmeidler J (1992) Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol 31(3):286–292
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5(11):863–873
Schenck JF, Zimmerman EA, Li Z, Adak S, Saha A, Tandon R et al (2006) High-field magnetic resonance imaging of brain iron in Alzheimer disease. Top Magn Reson Imaging 17(1):41–50
Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA et al (2007) Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68(21):1820–1825
Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP et al (2009) Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology 253(2):497–504
Pujol J, Junqué C, Vendrell P, Grau JM, Martí-Vilalta JL, Olivé C et al (1992) Biological significance of iron-related magnetic resonance imaging changes in the brain. Arch Neurol 49(7):711–717
Sullivan EV, Adalsteinsson E, Rohlfing T, Pfefferbaum A (2009) Relevance of iron deposition in deep gray matter brain structures to cognitive and motor performance in healthy elderly men and women: exploratory findings. Brain Imaging Behav 3(2):167–175
Bartzokis G, Lu PH, Tingus K, Peters DG, Amar CP, Tishler TA et al (2011) Gender and iron genes may modify associations between brain iron and memory in healthy aging. Neuropsychopharmacology 36(7):1375–1384
Penke L, Valdés Hernandéz MC, Maniega SM, Gow AJ, Murray C, Starr JM et al (2012) Brain iron deposits are associated with general cognitive ability and cognitive aging. Neurobiol Aging 33(3):510–517
Rodrigue KM, Daugherty AM, Haacke EM, Raz N (2012) The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb Cortex, DOI: 10.1093/cercor/bhs139
Brass SD, Benedict RH, Weinstock-Guttman B, Munschauer F, Bakshi R (2006) Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult Scler 12(4):437–444
House MJ, St Pierre TG, Foster JK, Martins RN, Clarnette R (2006) Quantitative MR imaging R2 relaxometry in elderly participants reporting memory loss. AJNR Am J Neuroradiol 27(2):430–439
Ding B, Chen KM, Ling HW, Sun F, Li X, Wan T et al (2009) Correlation of iron in the hippocampus with MMSE in patients with Alzheimer's disease. J Magn Reson Imaging 29(4):793–798
Fredriksson A, Schröder N, Eriksson P, Izquierdo I, Archer T (1999) Neonatal iron exposure induces neurobehavioural dysfunctions in adult mice. Toxicol Appl Pharmacol 159(1):25–30
Schröder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T (2001) Memory deficits in adult rats following postnatal iron administration. Behav Brain Res 124(1):77–85
de Lima MN, Polydoro M, Laranja DC, Bonatto F, Bromberg E, Moreira JC et al (2005) Recognition memory impairment and brain oxidative stress induced by postnatal iron administration. Eur J Neurosci 21(9):2521–2528
Schröder N, Figueiredo LS, de Lima MNM (2013) Role of brain iron accumulation in cognitive dysfunction: evidence from animal models and human studies. J Alzheimers Dis 34(4):797–812
Liu W, Tian F, Kurata T, Morimoto N, Abe K (2012) Dynamic changes of mitochondrial fusion and fission proteins after transient cerebral ischemia in mice. J Neurosci Res 90(6):1183–1189
Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X (2009) The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem 109(1):153–159
Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA (2003) Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol 15(6):706–716
Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75(5):762–777
Shimada A, Keino H, Satoh M, Kishikawa M, Hosokawa M (2003) Age-related loss of synapses in the frontal cortex of SAMP10 mouse: a model of cerebral degeneration. Synapse 48(4):198–204
Kajta M (2004) Apoptosis in the central nervous system: Mechanisms and protective strategies. Pol J Pharmacol 56(6):689–700
Hampson AJ, Grimaldi M, Axelrod J, Wink D (1998) Cannabidiol and (−) Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A 95(14):8268–8273
García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, Fernández-Ruiz J (2007) Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res 1134(1):162–170
Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J (2010) The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis 37(2):434–440
Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA (2004) Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem 89(1):134–141
Pazos MR, Cinquina V, Gómez A, Layunta R, Santos M, Fernández-Ruiz J et al (2012) Cannabidiol administration after hypoxia–ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 63(5):776–783
Sagredo O, Ramos JA, Decio A, Mechoulam R, Fernández-Ruiz J (2007) Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci 26(4):843–851
Fagherazzi EV, Garcia VA, Maurmann N, Bervanger T, Halmenschlager LH, Busato SB et al (2012) Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology (Berlin) 219(4):1133–1140
Karl T, Cheng D, Garner B, Arnold JC (2012) The therapeutic potential of the endocannabinoid system for Alzheimer's disease. Expert Opin Ther Targets 16(4):407–420
Silva PF, Garcia VA, da Dornelles AS, Silva VK, Maurmann N, Portal BC et al (2012) Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience 200:42–49
Arciello M, Capo CR, Cozzolino M, Ferri A, Nencini M, Carrì MT et al (2010) Inactivation of cytochrome c oxidase by mutant SOD1s in mouse motoneuronal NSC-34 cells is independent from copper availability but is because of nitric oxide. J Neurochem 112(1):183–192
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Amaral AU, Seminotti B, Cecatto C, Fernandes CG, Busanello EN, Zanatta A et al (2012) Reduction of Na(+), K(+)-ATPase activity and expression in cerebral cortex of glutaryl-CoA dehydrogenase deficient mice: A possible mechanism for brain injury in glutaric aciduria type I. Mol Genet Metab 107(3):375–382
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Bonefeld BE, Elfving B, Wegener G (2008) Reference genes for normalization: a study of rat brain tissue. Synapse 62:302–309
Kar R, Mishra N, Singha PK, Venkatachalam MA, Saikumar P (2010) Mitochondrial remodeling following fission inhibition by 15d-PGJ2 involves molecular changes in mitochondrial fusion protein OPA1. Biochem Biophys Res Commun 399(4):548–554
Loucks FA, Schroeder EK, Zommer AE, Hilger S, Kelsey NA, Bouchard RJ et al (2009) Caspases indirectly regulate cleavage of the mitochondrial fusion GTPase OPA1 in neurons undergoing apoptosis. Brain Res 1250:63–74
Evans LC, Liu H, Thompson LP (2012) Differential effect of intrauterine hypoxia on caspase 3 and DNA fragmentation in fetal guinea pig hearts and brains. Reprod Sci 19(3):298–305
Manczak M, Reddy PH (2012) Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet 21(11):2538–2547
Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y et al (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105(49):19318–19323
Horowitz MP, Greenamyre JT (2010) Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis 20(Suppl 2):S551–S568
Dal-Pizzol F, Klamt F, Frota ML, Andrades ME, Caregnato FF, Vianna M et al (2001) Neonatal iron exposure induces oxidative stress in adult Wistar rat. Brain Res Dev Brain Res 130:109–114
Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ (1997) Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol 56(8):933–944
Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr et al (2005) Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis 7(2):103–117
Youle RJ, Karbowski M (2005) Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6(8):657–663
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125(7):1241–1252
Miwa CP, de Lima MN, Scalco F, Vedana G, Mattos R, Fernandez LL et al (2011) Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox Res 19(4):527–535
Salvador GA, Oteiza PI (2011) Iron overload triggers redox-sensitive signals in human IMR-32 neuroblastoma cells. Neurotoxicology 32(1):75–82
Avramovich-Tirosh Y, Reznichenko L, Mit T, Zheng H, Fridkin M, Weinreb O et al (2007) Neurorescue activity, APP regulation and amyloid-beta peptide reduction by novel multi-functional brain permeable iron-chelating-antioxidants, M-30 and green tea polyphenol, EGCG. Curr Alzheimer Res 4(4):403–411
Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G et al (2011) Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend 114(2–3):242–245
Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S et al (2013) Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 27(1):19–27
Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci 29(7):2053–2063
LeVine SM, Bilgen M, Lynch SG (2013) Iron accumulation in multiple sclerosis: an early pathogenic event. Expert Rev Neurother 13(3):247–250
de Lima MN, Presti-Torres J, Garcia VA, Guimarães MR, Scalco FS, Roesler R et al (2008) Amelioration of recognition memory impairment associated with iron loading or aging by the type 4-specific phosphodiesterase inhibitor rolipram in rats. Neuropharmacology 55(5):788–792
Perez VP, de Lima MN, da Silva RS, Dornelles AS, Vedana G, Bogo MR et al (2010) Iron leads to memory impairment that is associated with a decrease in acetylcholinesterase pathways. Curr Neurovasc Res 7(1):15–22
Acknowledgments
V.K.S. is supported by a CAPES/MEC fellowship. L.F. is supported by a FAPERGS scholarship. M.R.B, J.E.H., A.W.Z., J.A.C., and N.S. are CNPq Research fellows. This research was supported by the National Institute for Translational Medicine (INCT-TM). This manuscript was reviewed by a professional science editor and by a native English-speaking copy editor to improve readability.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, V.K., de Freitas, B.S., da Silva Dornelles, A. et al. Cannabidiol Normalizes Caspase 3, Synaptophysin, and Mitochondrial Fission Protein DNM1L Expression Levels in Rats with Brain Iron Overload: Implications for Neuroprotection. Mol Neurobiol 49, 222–233 (2014). https://doi.org/10.1007/s12035-013-8514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8514-7