Abstract
Although intracranial pressure (ICP) is essential to guide management of patients suffering from acute brain diseases, this signal is often neglected outside the neurocritical care environment. This is mainly attributed to the intrinsic risks of the available invasive techniques, which have prevented ICP monitoring in many conditions affecting the intracranial homeostasis, from mild traumatic brain injury to liver encephalopathy. In such scenario, methods for non-invasive monitoring of ICP (nICP) could improve clinical management of these conditions. A review of the literature was performed on PUBMED using the search keywords ‘Transcranial Doppler non-invasive intracranial pressure.’ Transcranial Doppler (TCD) is a technique primarily aimed at assessing the cerebrovascular dynamics through the cerebral blood flow velocity (FV). Its applicability for nICP assessment emerged from observation that some TCD-derived parameters change during increase of ICP, such as the shape of FV pulse waveform or pulsatility index. Methods were grouped as: based on TCD pulsatility index; aimed at non-invasive estimation of cerebral perfusion pressure and model-based methods. Published studies present with different accuracies, with prediction abilities (AUCs) for detection of ICP ≥20 mmHg ranging from 0.62 to 0.92. This discrepancy could result from inconsistent assessment measures and application in different conditions, from traumatic brain injury to hydrocephalus and stroke. Most of the reports stress a potential advantage of TCD as it provides the possibility to monitor changes of ICP in time. Overall accuracy for TCD-based methods ranges around ±12 mmHg, with a great potential of tracing dynamical changes of ICP in time, particularly those of vasogenic nature.
Similar content being viewed by others
Introduction
Intracranial pressure (ICP) is an important monitoring modality in the clinical management of several neurological diseases carrying intrinsic risk of potentially lethal intracranial hypertension (ICH). ICP essentially consists of four components, driven by different physiological mechanisms [1]: inflow and volume of arterial blood, venous blood outflow, cerebrospinal fluid (CSF) circulation, and brain volume. These components are responsible for different patterns of ICH.
Although ICP can guide patient management in neurocritical care, it is not commonly monitored in many clinical conditions outside this environment. The invasive character of the standard methods for ICP monitoring (epidural, subdural, intraparenchymal, and intraventricular monitors) and their associated risks to the patient (infections, brain tissue lesions, and hemorrhage) contribute to this scenario. They have prevented ICP monitoring in a broad range of diseases like in patients with risk of coagulopathy, as well as in other conditions in which invasive monitoring is not considered or outweighed by the risks of the procedure. Another downside is related to costs and availability: invasive monitoring is an expensive technique, requires trained personnel and neurosurgical settings. Average cost of intraparenchymal microtransducer is US $600, additionally to US $6000–10,000 for the display monitor [2]. Provided that knowledge of ICP can be crucial for the successful management of patients in many sub-critical conditions, non-invasive estimation of ICP (nICP) may be helpful when indications for invasive ICP monitoring are not met and when it is not immediately available or even contraindicated.
Transcranial Doppler Ultrasonography (TCD) was first described by Aaslid et al. [3]. Apart from many clinical applications, TCD waveform analysis has been investigated as a technique for nICP estimation, and this could represent one of its most useful applications outside the critical care environment. It is conceivable if one considers that increased ICP could affect the waveform of blood flow velocity in major cerebral vessels which have compliant walls. Such vessels are subjected to an external pressure (ICP) and an internal pressure (arterial blood pressure—ABP). Active tension of the arterial walls and the arterial wall compliance are another (and unknown) parameters, which undoubtedly fall into the equation. On top of this, not only all changes in FV waveform, like low diastolic cerebral blood flow velocity (FVd), peaked waveform, and higher pulsatility index (PI) values can be observed with TCD during elevated ICP, but also in arterial hypotension and hypocapnia [4, 5].
TCD-based nICP methods are mainly based on approximate semi-quantitative relationships between cerebrovascular dynamics and ICP. They can be divided into three categories: (I) methods based on the TCD-derived pulsatility index; (II) methods based on the calculation of non-invasive cerebral perfusion pressure (nCPP); and (III) methods based on mathematical models.
Although derived from the same principle, there is a considerable variability in the reported accuracy of these methods inter- and intra-categories.
Considering the wide range of TCD applications as a technique for nICP monitoring, the purpose of this review is to generally present these methods and their documented clinical or experimental applications with measures of accuracy.
Methods
A review of the literature was performed on PUBMED database using the search keywords ‘Transcranial Doppler non-invasive intracranial pressure.’ Works from 1985 to 2015 were found, in a total of 98 studies. The inclusion criteria were the use of Transcranial Doppler as tool for non-invasive ICP with clinical or experimental applications of such methods. Excluded papers consisted of works using TCD, but with no application for non-invasive ICP estimation, or absence of clinical or experimental applications in papers describing methods using TCD for non-invasive ICP estimation. The selected articles were then subdivided into the three categories aforementioned. Only full-length available articles in the English language were considered (see flow diagram in Fig. 1). The total number of articles considered was 37, with occasional repetitions within each nICP category, i.e., certain articles presented assessments in more than one category.
Results for each nICP category are presented in Tables 1, 2, and 3. They include main findings and measures of accuracy of the studies considered. Sections in Results present major attributes of the nICP categories.
Results
Methods Based on the Correlation Between ICP and PI (nICP_PI)
Pulsatility index describes quantitative and qualitative changes in the morphology of the TCD waveform resulting from cerebral perfusion pressure changes. It represents a relationship between the difference of systolic flow velocity (FVs) and FVd divided by mean flow velocity (FVm). All possible methods based on TCD-derived pulsatility index are based on observation that ICP and PI are positively correlated during increases of ICP. However, increase in PI is not specific for increase in ICP. In certain situations, such as a drop in CPP, PI presents an increasing trend, which can be related to increases in ICP or decreases in ABP (Fig. 2). The same behavior occurs during decrease in PaCO2 (partial pressure of carbon dioxide) or increase in pulsatility of ABP waveform. Mathematically, PI can be expressed as inversely proportional to mean CPP, directly proportional to pulse amplitude of arterial blood pressure and non-linearly proportional to the compliance of the arterial bed (Ca), cerebrovascular resistance (CVR), and heart rate (HR) [6].
PI behavior during drop in CPP observed in a traumatic brain-injured patient (source: Brain Physics Laboratory TBI Database, University of Cambridge). Dashed lines represent periods when PI increased due to increase in ICP, independently of changes in ABP. CPP cereberal perfusion pressure, PI pulsatility index, ICP intracranial pressure, ABP arterial blood pressure, TBI traumatic brain injury
Table 1 presents the papers which studied the relationship between PI and ICP. Accuracy of nICP estimation varies from ±5 to ±43.8 mmHg. The most favorable results are from Bellner et al. [7], in which the authors found a 95 % confidence interval for prediction of ±4.2 mmHg and strong correlation coefficient with ICP, R = 0.94 (p < 0.05). However, such results were never replicated by other authors.
Methods Based on Estimation of CPP
The second approach for nICP monitoring was primarily intended for estimating the nCPP. However, non-invasive ICP can be calculated based on the assumption that nICP = ABP − nCPP. Four methods are described in the literature (Table 2).
nICP_Aaslid
Aaslid et al. [8] have determined CPP based on the amplitudes of the fundamental frequency components of FV (F) and of the arterial blood pressure (A):
nICP_FVd
Some studies have demonstrated that specific patterns of TCD waveform, such as a decrease in diastolic flow velocity, reflect impaired cerebral perfusion caused by a decrease in CPP [9, 10] (Fig. 3). Based on waveform analysis of FV [10], the proposed equation was
14 mmHg is a calibration (zeroing) parameter established for traumatic brain injury patients.
Systolic and diastolic flow velocities behavior during a drop of cerebral perfusion pressure during a plateau wave increase in ICP observed in a traumatic brain-injured patient (source: Brain Physics Laboratory TBI Database, University of Cambridge). FVd component in this case indicates inadequate cerebral perfusion. CPP cerebral perfusion pressure, FVs systolic flow velocity, FVd diastolic flow velocity, ICP intracranial pressure, TBI traumatic brain injury
nCPP_Edouard
This method is based on the combination of the phasic values of both FV and ABP. The non-invasive CPP (nCPP) was calculated using the following formula [11]:
where ABPm and ABPd are the mean and diastolic ABP, respectively.
nICP_CrCP
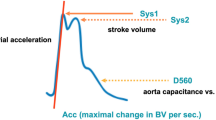
Critical closing pressure (CrCP) represents a threshold of ABP, below which the blood pressure in the brain microvasculature is inadequate to prevent the collapse and cessation of blood flow [12]. CrCP equals the sum of ICP and vascular wall tension (WT) [12, 13]: CrCP = ICP + WT. Given the association of this parameter with the vasomotor tone of small blood vessels (i.e., wall tension), CrCP may be able to provide information regarding the state of cerebral haemodynamics in several neurological conditions [12, 14–17] and then could reflect changes in CPP (Fig. 4). The equation for nCPP estimation based on CrCP is
Constant coefficients (0.734, 0.266, 7.026) were derived from analysis of database of 232 TBI retrospective cases [18]. CaBV Amp represents the fundamental amplitude of the cerebral arterial blood volume. ABP Amp represents the fundamental amplitude of arterial blood pressure.
Representation of the CrCP interaction with ICP and WT in a situation of intracranial hypertension observed in a traumatic brain-injured patient (source: Brain Physics Laboratory TBI Database, University of Cambridge). During the increase of ICP, the CrCP also increases and WT decreases as an effect of preserved autoregulation. ABP arterial blood pressure, CrCP critical closing pressure, ICP intracranial pressure, WT wall tension, TBI traumatic brain injury
The accuracy for these methods was mainly reported for nCPP estimations and varied from ±12 to 48.9 mmHg. Variability for nICP in this case ranged from ±9.19 to ±59.60 mmHg. Out of the four methods, the best accuracy for prediction ICP was reported by Cardim et al. [19] elsewhere, using the nICP_CrCP, in which ICP could be predicted within a confidence interval of ±9.19 mmHg.
Model-based nICP Methods
Black-Box Model for Estimation of ICP (nICP_BB)
In this model, the intracranial compartment was considered a black-box (BB) system, with ICP being a system response to the incoming signal ABP [20]. The system response was described in terms of a transfer function between ABP and ICP [21, 22]. The transfer function was controlled by TCD and ABP derived parameters, the so-called TCD characteristics, which include ICP-related parameters and an ABP to TCD transfer function. The rules of this TCD-based linear control had been formerly determined using a multiple regression model between TCD characteristics and ABP-ICP transfer function on datasets of reference patients. The output data provide continuous full waveform of nICP (in mmHg) (Fig. 5).
Schematic representation of the black-box model (Schmidt et al. [20]), for nICP estimation. A known transfer function (represented by a linear model) between ABP and FV, alongside modification (TCD) characteristics are used as means to dynamically define the rules for a transformation of ABP into nICP (unknown transfer function—a linear model between ABP and ICP). ABP arterial blood pressure, FV cerebral blood flow velocity, TCD transcranial Doppler, nICP non-invasive intracranial pressure
Application of this model is summarized in Table 3.
Cerebrovascular Dynamics Model for Estimation of ICP (nICP_Heldt [23, 24])
This model-based nICP method focuses on the major intracranial compartments and their associated variables: brain tissue, cerebral vasculature, and cerebrospinal fluid (CSF). It continuously estimates and tracks ICP using measurements of peripheral ABP and FV in the middle cerebral artery (MCA). This physiological model of cerebrovascular dynamics is represented by a circuit analog and provides mathematical limits that relate the measured waveforms to ICP. Patient-specific ICP estimations are produced by an algorithm, with no calibration or training in specific populations needed. The dynamical model of CSF and cerebral blood circulation has been first published by Ursino and Lodi [25].
Accuracy of this method is summarized in Table 3.
Non-linear Regressions
Modified Black-Box Model
The previously described black-box model for ICP estimation [20] adopts a linear relationship among ABP, ICP, and FV. Xu et al. [26] assuming that the relationships among these three signals are more complex than linear models, and consequently not adequate to depict the relationship between f and w coefficients (relationship between ABP and ICP and that between ABP and FV, respectively), investigated the adoption of several nonlinear regression approaches. Considering that nonlinear regressions such as support vector machines (SVMs) [27], kernel spectral regression (KSR) [28] have been proved to be more powerful for the prediction problem than the linear ones [29, 30], the authors proposed to use these approaches to model the relationship between coefficients f and w.
The ICP estimation showed that the mean ICP error by the nonlinear approaches can be reduced compared to the original approach (Table 3). Statistical tests also demonstrated that the ICP estimation error by the proposed nonlinear kernel approaches is statistically smaller than that obtained with nICP_BB.
Data Mining
Hu et al. [31] initially proposed an innovative data mining framework of nICP assessment. The proposed framework explores the rules of deriving ICP from ABP and FV that are captured implicitly by a signal database without using a mathematical model. The main strategy of the this framework is to provide a mapping function to quantify the uncertainty of an ICP estimate associated with each database entry, and to use this information to determine the best entry to build an ICP simulation model for an optimal ICP estimation. In comparison to Schmidt’s method (nICP_BB), for example, this model presented a smaller median normalized prediction error (bias), and a greater median correlation coefficient between estimated and measured normalized ICP (Table 3).
In another work of the same group, Kim et al. [32] aimed at adopting a new (linear and nonlinear) mapping functions into the previous data mining framework for nICP estimation to demonstrate that the performance of nICP assessment could be improved by utilizing proper mapping functions. Results are summarized in Table 3.
Semisupervised Learning
As previously seen, FV waveform analysis has been frequently applied for non-invasive ICP assessment. Kim et al. [33] introduced a non-invasive detection of intracranial hypertension method based on the TCD measurement of FV alone to demonstrate its performance both in the supervised and semisupervised learning settings (Table 3).
Out of these five model-based methods, the best accuracy was reported by Kashif and Heldt et al. [24], in which authors present a strong correlation between nICP and ICP of R = 0.90, sensitivity of 83 %, specificity of 70 %, with an AUC of 0.83 for detecting ICP ≥ 20 mmHg. In addition, a 95 % CI for prediction of ICP of ±14.9 mmHg (SDE of ±7.6 mmHg) was found for this method. Although other reviewed methods presented smaller 95 % CI, considering all measures of accuracy together, nICP_Heldt was the one showing the best performance.
Discussion
Intracranial pressure and its management have been considered of fundamental importance in the treatment of neurocritical patients. ICP monitoring has been available since 1951, but it is important to realize that the monitor itself contributes little to outcome without proper interpretation and secondary analysis of the observed signal [34]. Instead, a positive outcome depends on how the data from the monitor are used and whether an effective treatments exists [34]. In a recent study, Chesnut et al., demonstrated that there was no difference in primary outcome in TBI patients who received ICP monitoring [35]. However, while this trial has internal validity, it has not been externally validated and did not test whether treatment of ICP per se makes a difference, but rather compared two management protocols (patients with or without ICP monitoring) [34]. Moreover, ICP should not be considered solely as a “number,” as waveform analysis of this parameter is also important [36]. For instance, ICP waveform analysis can provide information on the state of cerebrovascular reactivity (PRx index), compensatory reserve (RAP index) and can be used to estimate individualized optimal cerebral perfusion pressure levels [37, 38].
Despite eventual complications that might raise from invasive monitoring, direct methods still remain as the gold standards [2]. When direct ICP monitoring is contraindicated, a reliable non-invasive method would be helpful, at least in the early stages of treatment, when it could act as a screening tool. Such scenario would be beneficial to a wide range of neurological conditions in which ICP monitoring is not usually applied or is a neglected parameter, such as cerebral malaria [39, 40], status epilepticus, mild or moderate TBI, brain tumors. For an example, TCD has been demonstrated to accurately screen patients with mild or moderate TBI at risk of secondary neurological deterioration [41].
The advent of Transcranial Doppler Ultrasonography allowed the development of several methods dedicated to assess the cerebrovascular circulation and dynamics, particularly non-invasive assessment of ICP and CPP. Moreover, subject to good fixing of ultrasound probes, it allows monitoring of these parameters as they may change in time. TCD comprises most of the features a nICP method should contemplate: relatively low cost, risk-free, easily available, portable, high temporal resolution, repeatable and suitable for emergency and ambulatory settings. Nevertheless, as most of the non-invasive techniques, TCD also presents some intrinsic disadvantages which can negatively influence its accuracy. They are mainly represented by signal transmission attenuation through the cranial bones, linearity and stability of the signal in time. Furthermore, TCD measurements may be especially difficult in a certain percentage of the population (up to 8 %) which does not present an adequate acoustic window for artery insonation [42]. On the other hand, accuracy may not be the primary performance measure in every clinical situation, and such downsides may be compensated for by the ability of the method to track changes and trends of ICP over time, rather than its absolute value.
In addition, a possible disadvantage about TCD is related to its principle of working. It is known that this technique is limited to detecting changes in the arterial bed of vasogenic origin [43] (i.e., changes of arterial blood volume). Considering that ICP consists of several components (i.e., inflow and volume of arterial blood, venous blood outflow, CSF circulation and brain parenchyma volume), it is expected that a TCD-based method would present lower accuracy if changes in ICP were caused, for example, by derangements in the CSF circulation or by increase in parenchyma volume, rather than if they were purely of vasogenic origin. This is mainly because changes in the CSF and brain parenchyma compartments would not be promptly transmitted to the arterial bed as of those of vasogenic origin. Such characteristic can be exemplified by the fact that in certain situations where changes of ICP related to vasogenic fluctuations (plateau waves, B waves) overlaps the changes of ICP related to CSF circulation (for instance during CSF infusion test), there is a strong correlation in time domain between real and TCD-estimated ICP (as seen in Fig. 6, obtained from Cardim et al. [44]), even showing reliable replications of vasogenic waves patterns.
Example of vasogenic waves during CSF infusion test (Cardim et al. [44]). Shadowed areas in (a) and (b) represent ICP waves of vasogenic origin. It is possible to observe that at least for trends in time, there were good correspondence between ICP and nICP methods; nICP_BB non-invasive ICP method based on mathematical black-box model [6]; nICP_FVd non-invasive ICP method based on FVd [10]; nICP_CrCP non-invasive ICP method based on CrCP [18]; nICP_PI non-invasive ICP method based on PI
According to the revision of the presented methods, the measures of accuracy for each method varied substantially within and among nICP categories as observed in Tables 1, 2 and 3. For the approaches based on mathematical models, for instance, the most frequent measures were the ‘Bias’ and ‘SDE’ (standard deviation of the error [bias]), and MAD (mean absolute difference).
Over measures of accuracy, a standard statistical assessment would be interesting for works on non-invasive intracranial pressure methods. For instance, at least for a clinical point of view, an assessment should contain the following statistical indicators: (I) correlation between nICP and measured ICP considering mean values of ICP and changes of ICP in time domain; (II) Bias and 95 % CI for prediction of ICP; (III) ROC analyses including the nICP method prediction ability at a certain threshold (usually around 20 mmHg for intracranial hypertension), sensitivity and specificity. Altogether, these parameters should provide the clinician a comprehensive picture of the qualities and downsides of a method.
Another aspect that might confuse interpretation and comparisons is how the nICP averages were obtained, i.e., every work present with different average calculation windows. The number of samples should also be considered when comparing such results. Moreover, information about sensitivity and specificity, confidence intervals for prediction or any of the above mentioned parameters is not systematically available in the majority of the reviewed papers. Some of the studies also include a small number of patients, making a quantitative comparison unfeasible. This variability illustrates the importance and necessity of studies applying the same number of samples, calculation methods and measures of accuracy in order to compare different nICP methods consistently.
The several degrees of approximation for ICP monitoring (i.e., epidural, subdural, intraparenchymal and intraventricular) could also contribute to a misleading interpretation when validating nICP methods against different invasive techniques. This is due to the presence of multiple intracranial compartments of variable deformability and ability to transmit pressure. Thus, ICP needs to be considered as an anisotropic parameter rather than a global isotropic pressure equally distributed in all intracranial compartments. Over this concept, each invasive method would then be specific to measuring compartmental pressures according to where they are located in the intracranial system. As proofs of this concept, simultaneous measurement of ICP by intraparenchymal and intraventricular probes showed a bias of −1.2 and a 95 % CI of ±6.8 mmHg (SDE of ±3.4 mmHg) [45]. In another study, simultaneous measurements of ICP using intraparenchymal and epidural probes presented a bias of 4.3 mmHg, with 95 % CI of ±17 mmHg (SDE of ±8.5 mmHg) [46]. Under these circumstances, the characteristics of invasive ICP monitoring should also be considered in the standard nICP assessment.
Nevertheless, qualitative-wise, TCD-based nICP methods generally presented a positive degree of agreement and acceptable correlations with measured ICP (or with CPP for nCPP-based methods), with exceptions for PI-based methods. For this category, even though most of the studies indicated a direct, or at least indirect, correlation between PI and ICP, there is a divergence whether PI can predict ICP reliably, with some studies showing rather weak or even inexistent correlations between these two parameters [6, 19, 47–51]. Such a controversy might originate from the different conditions in which PI can increase independently of increases in ICP.
Considering the 95 % CI for the presented methods, there is a wide variability for the different method categories (from ±4.2 to ±59.60 mmHg). The authors’ personal experience on working with TCD-based methods suggests there is an overall intrinsic confidence interval of around ±12 mmHg, which still needs to be extensively validated in different patient populations and clinical conditions. Provided that the clinically relevant range of ICP is about 10 or 20 mmHg, TCD-based methods at the current state of development are not able to predict mean values of ICP with great confidence. However, the cerebral circulation dynamics can be observed with such methods as nICP changes in time domain, and tracked in real-time in the clinical setting (as observed in Fig. 6, for instance). This is one of the advantages of Transcranial Doppler Ultrasonography and may become particularly useful as a primary assessment tool in centres where ICP measurements are not routinely applied, or in patients in whom ICP monitoring is unavailable or may not be clearly indicated.
In conclusion, although Transcranial Doppler Ultrasonography consists of a technique with various possibilities for nICP estimation, there is still a necessity of studies to systematically compare them in different clinical conditions, in order to determine which approach offers the best reliability to monitor ICP dynamics non-invasively.
References
Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry 2004;75:813–21. http://jnnp.bmj.com/cgi/doi/10.1136/jnnp.2003.033126.
Surgeons. BTFAA of NSC of N. Guidelines for the management of severe traumatic brain injury 3rd Edition. J Neurosurg [Internet] 2007;24(Suppl):S1–106. http://www.ncbi.nlm.nih.gov/pubmed/17511534.
Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769–74.
Klingelhöfer J, Conrad B, Benecke R, Sander D, Markakis E. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol. 1988;235(3):159–62.
Czosnyka M, Richards HK, Whitehouse HE, Pickard JD. Relationship between transcranial Doppler-determined pulsatility index and cerebrovascular resistance: an experimental study. J Neurosurg. 1996;84(1):79–84.
De Riva N, Budohoski KP, Smielewski P, et al. Transcranial doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66.
Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62:45–51.
Miller JD, Teasdale GM, Rowan JO, et al. Estimation of cerebral perfusion pressure from arterial blood pressure and transcranial Doppler recordings. In: Miller JD, Teasdale GM, Rowan JO, editors. Intracranial pressure VI. Berlin: Springer; 1986. p. 226–9.
Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S. The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg. 1992;77(1):55–61.
Czosnyka M, Matta BF, Smielewski P, Kirkpatrick PJ, Pickard JD. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg. 1998;88:802–8.
Edouard AR, Vanhille E, Le-Moigno S, Benhamou D, Mazoit J-X. Non-invasive assessment of cerebral perfusion pressure in brain injured patients with moderate intracranial hypertension. Br J Anaesth. 2005;94(2):216–21. http://bja.oxfordjournals.org/cgi/content/long/94/2/216.
Nichol J, Girling F, Jerrard W, Claxton EB, Burton AC. Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am J Physiol. 1951;164(2):330–44. http://www.ncbi.nlm.nih.gov/pubmed/14810938.
Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodynamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg. 1974;41(5):597–606. http://www.ncbi.nlm.nih.gov/pubmed/4214313.
Czosnyka M, Richards H, Pickard JD, Harris N, Iyer V. Frequency-dependent properties of cerebral blood transport—an experimental study in anaesthetized rabbits. Ultrasound Med Biol. 1994;20(4):391–9.
Michel E, Hillebrand S, vonTwickel J, Zernikow B, Jorch G. Frequency dependence of cerebrovascular impedance in preterm neonates: a different view on critical closing pressure. J Cereb Blood Flow Metab. 1997;17(10):1127–31.
Puppo C, Camacho J, Yelicich B, Moraes L, Biestro A, Gomez H. Bedside study of cerebral critical closing pressure in patients with severe traumatic brain injury: a transcranial Doppler study. Acta Neurochir Suppl. 2012;114:283–8.
Varsos GV, Richards H, Kasprowicz M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab. 2013;33(2):235–43. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3564193&tool=pmcentrez&rendertype=abstract.
Varsos GV, Kolias AG, Smielewski P, et al. A noninvasive estimation of cerebral perfusion pressure using critical closing pressure. J Neurosurg. 2015;123:638–48.
Cardim D, Robba C, Donnelly J, et al. Prospective study on non-invasive assessment of ICP in head injured patients: comparison of four methods. J Neurotrauma 2015. http://www.ncbi.nlm.nih.gov/pubmed/26414916.
Schmidt B, Klingelhofer J, Md J, Schwarze JJ, Sander D, Wittich I. Noninvasive prediction of intracranial pressure curves using transcranial Doppler ultrasonography and blood pressure curves. Stroke. 1997;28:2465–72.
Kasuga Y, Nagai H, Hasegawa Y, Nitta M. Transmission characteristics of pulse waves in the intracranial cavity of dogs. J Neurosurg. 1987;66(6):907–14.
Marmarelis PMV. Analysis of physiological systems. New York: Plenum Press; 1978.
Kashif FM, Heldt T, Verghese GC. Model-based estimation of intracranial pressure and cerebrovascular autoregulation. Comput Cardiol. 2008;35:369–72.
Kashif FM, Verghese GC, Novak V, Czosnyka M, Heldt T. Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med. 2012;4:129ra44.
Ursino M, Lodi CA. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J Appl Physiol. 1997;82(4):1256–69. http://www.ncbi.nlm.nih.gov/pubmed/9104864.
Xu P, Kasprowicz M, Bergsneider M, Hu X. Improved noninvasive intracranial pressure assessment with nonlinear kernel regression. IEEE Trans Inf Technol Biomed. 2010;14(4):971–8.
Lin C-J. Formulations of support vector machines: a note from an optimization point of view. Neural Comput. 2001;13(2):307–17.
Cai D, He X, Han J. Spectral regression for efficient regularized subspace learning. In: Proceedings of the IEEE international conference on computer vision (2007).
Melgani F, Bazi Y. Classification of electrocardiogram signals with support vector machines and particle swarm optimization. IEEE Trans Inf Technol Biomed. 2008;12(5):667–77.
Douglas M, Bates DGW. Nonlinear regression analysis and its applications. New York: Wiley; 2007. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0470139005.html.
Hu X, Nenov V, Bergsneider M, Martin N. A data mining framework of noninvasive intracranial pressure assessment. Biomed Signal Process Control. 2006;1(1):64–77.
Kim S, Scalzo F, Bergsneider M, Vespa P, Martin N, Hu X. Noninvasive intracranial pressure assessment based on a data-mining approach using a nonlinear mapping function. IEEE Trans Biomed Eng. 2012;59(3):619–26.
Kim S, Hamilton R, Pineles S, Bergsneider M, Hu X. Noninvasive intracranial hypertension detection utilizing semisupervised learning. IEEE Trans Biomed Eng. 2013;60(4):1126–33.
Chesnut R, Videtta W, Vespa P, Le Roux P. Intracranial pressure monitoring: fundamental considerations and rationale for monitoring. Neurocrit Care 2014;21: 64–84. http://www.ncbi.nlm.nih.gov/pubmed/25208680.
Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med 2012;367(26):2471–81. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3565432&tool=pmcentrez&rendertype=abstract.
Czosnyka M, Smielewski P, Timofeev I, et al. Intracranial pressure: more than a number. Neurosurg Focus. 2007;22(5):E10.
Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16(2):258–66.
Aries MJH, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40(8):2456–63.
Idro R, Marsh K, John CC, Newton CRJ. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68(4):267–74.
Newton CR, Hien TT, White N. Cerebral malaria. J Neurol Neurosurg Psychiatry 2000;69(4):433–41. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1737146&tool=pmcentrez&rendertype=abstract.
Bouzat P, Francony G, Declety P, et al. Transcranial Doppler to screen on admission patients with mild to moderate traumatic brain injury. Neurosurgery. 2011;68(6):1603–9.
Maeda H, Matsumoto M, Handa N, et al. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial Doppler method. Stroke. 1993;24(5):670–5.
Sloan MA, Alexandrov AV, Tegeler CH, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62(9):1468–81.
Cardim D, Czosnyka M, Donnelly J, et al. Assessment of non-invasive ICP during CSF infusion test: an approach with transcranial Doppler. Acta Neurochir. (Wien) 2016;158(2):279–87. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4715127&tool=pmcentrez&rendertype=abstract.
Koskinen LOD, Olivecrona M. Clinical experience with the intraparenchymal intracranial pressure monitoring Codman microsensor system. Neurosurgery. 2005;56(4):693–7.
Eide PK, Sorteberg W. Simultaneous measurements of intracranial pressure parameters in the epidural space and in brain parenchyma in patients with hydrocephalus. J Neurosurg. 2010;113(6):1317–25. http://www.ncbi.nlm.nih.gov/pubmed/20799859.
Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009;72(4):389–94. doi:10.1016/j.surneu.2009.02.012.
Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery 2012;71(4):853–61. http://www.ncbi.nlm.nih.gov/pubmed/22791038.
Behrens A, Lenfeldt N, Ambarki K, Malm J, Eklund A, Koskinen LO. Transcranial doppler pulsatility index: not an accurate method to assess intracranial pressure. Neurosurgery. 2010;66(6):1050–7.
Hanlo PW, Gooskens RHJM, Nijhuis IJM, et al. Value of transcranial Doppler indices in predicting raised ICP in infantile hydrocephalus—a study with review of the literature. Child’s Nerv Syst. 1995;11(10):595–603.
Rainov NG, Weise JB, Burkert W. Transcranial Doppler sonography in adult hydrocephalic patients. Neurosurg Rev. 2000;23:34–8.
Steiger HJ. Carotid Doppler hemodynamics in posttraumatic intracranial hypertension. Surg Neurol. 1981;16(6):459–61. http://www.ncbi.nlm.nih.gov/pubmed/7330769.
Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S. The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg. 1992;77(1):55–61. http://www.ncbi.nlm.nih.gov/pubmed/1607972.
Homburg AM, Jakobsen M, Enevoldsen E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol Scand. 1993;87(6):488–93.
Martin NA, Patwardhan RV, Alexander MJ, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87(1):9–19.
McQuire JC, Sutcliffe JC, Coats TJ. Early changes in middle cerebral artery blood flow velocity after head injury. J Neurosurg. 1998;89(4):526–32.
Moreno JA, Mesalles E, Gener J, et al. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;8(1):e8.
Asil T, Uzunca I, Utku U, Berberoglu U. Monitoring of increased intracranial pressure resulting from cerebral edema with transcranial Doppler sonography in patients with middle cerebral artery infarction. J Ultrasound Med. 2003;22:1049–53.
Voulgaris SG, Partheni M, Kaliora H, Haftouras N, Pessach IS, Polyzoidis KS. Early cerebral monitoring using the transcranial Doppler pulsatility index in patients with severe brain trauma. Med Sci Monit. 2005;11(2):CR49–52.
Brandi G, Béchir M, Sailer S, Haberthür C, Stocker R, Stover JF. Transcranial color-coded duplex sonography allows to assess cerebral perfusion pressure noninvasively following severe traumatic brain injury. Acta Neurochir (Wien). 2010;152(6):965–72.
Melo JRT, Di-Rocco F, Blanot S, et al. Transcranial Doppler can predict intracranial hypertension in children with severe traumatic brain injuries. Childs Nerv Syst. 2011;27(6):979–84. http://www.ncbi.nlm.nih.gov/pubmed/21207041.
Wakerley BR, Kusuma Y, Yeo LLL, et al. Usefulness of transcranial doppler-derived cerebral hemodynamic parameters in the noninvasive assessment of intracranial pressure. J Neuroimaging. 2014;25:111–6.
Wakerley B, Yohana K, Luen Teoh H, Tan CW, Chan BPL, Sharma VK. Non-invasive intracranial pressure monitoring with transcranial Doppler in a patient with progressive cerebral venous sinus thrombosis. J Neuroimaging 2014;24(3):302–4. http://www.ncbi.nlm.nih.gov/pubmed/23228062.
O’Brien NF, Maa T, Reuter-Rice K. Noninvasive screening for intracranial hypertension in children with acute, severe traumatic brain injury. J Neurosurg Pediatr. 2015;16:420–25. http://www.ncbi.nlm.nih.gov/pubmed/26140576.
Robba C, Donnelly J, Bertuetti R, et al. Doppler non-invasive monitoring of ICP in an animal model of acute intracranial hypertension. Neurocrit Care 2015. http://www.ncbi.nlm.nih.gov/pubmed/26268137.
Schmidt EA, Czosnyka M, Gooskens I, et al. Preliminary experience of the estimation of cerebral perfusion pressure using transcranial Doppler ultrasonography. J Neurol Neurosurg Psychiatry. 2001;70(2):198–204.
Gura M, Silav G, Isik N, Elmaci I. Noninvasive estimation of cerebral perfusion pressure with transcranial doppler ultrasonography in traumatic brain injury. Turk Neurosurg. 2012;22(4):411–5.
Schmidt B, Czosnyka M, Schwarze JJ, et al. Evaluation of a method for noninvasive intracranial pressure assessment during infusion studies in patients with hydrocephalus. J Neurosurg. 2000;92(5):793–800.
Schmidt B, Czosnyka M, Klingelhöfer J. Clinical applications of a non-invasive ICP monitoring method. Eur J Ultrasound 2002;16(1–2):37–45. http://www.sciencedirect.com/science/article/pii/S0929826602000447.
Schmidt B, Czosnyka M, Raabe A, et al. Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003;34(1):84–9.
Acknowledgments
Cambridge Commonwealth, European & International Trust Scholarship (University of Cambridge) provided financial support in the form of Scholarship funding for DC. Woolf Fisher Trust provided financial support in the form of Scholarship funding for JD. Gates Cambridge Trust provided financial support in the form of Scholarship funding for XL. CNPQ provided financial support in the form of Scholarship funding for BCTC (Research Project 203792/2014-9). NIHR Brain Injury Healthcare Technology Co-operative, Cambridge, UK provided financial support in the form of equipment funding for DC, BC, and MC. The sponsors had no role in the design or conduct of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus (exception: MC occasionally lectures for Integra Life Science); membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), and no non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cardim, D., Robba, C., Bohdanowicz, M. et al. Non-invasive Monitoring of Intracranial Pressure Using Transcranial Doppler Ultrasonography: Is It Possible?. Neurocrit Care 25, 473–491 (2016). https://doi.org/10.1007/s12028-016-0258-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0258-6