Abstract
Background
Subarachnoid hemorrhage (SAH) is an independent prognostic indicator of outcome in adult severe traumatic brain injury (sTBI). There is a paucity of investigations on SAH in pediatric sTBI. The goal of this study was to determine in pediatric sTBI patients SAH prevalence, associated factors, and its relationship to short-term outcome.
Methods
We retrospectively analyzed 171 sTBI patients (pre-sedation GCS ≤8 and head MAIS ≥4) who underwent CT head imaging within the first 24 h of hospital admission. Data were analyzed with both univariate and multivariate techniques.
Results
SAH was found in 42 % of sTBI patients (n = 71/171), and it was more frequently associated with skull fractures, cerebral edema, diffuse axonal injury, contusion, and intraventricular hemorrhage (p < 0.05). Patients with SAH had higher Injury Severity Scores (p = 0.032) and a greater frequency of fixed pupil(s) on admission (p = 0.001). There were no significant differences in etiologies between sTBI patients with and without SAH. Worse disposition occurred in sTBI patients with SAH, including increased mortality (p = 0.009), increased episodes of central diabetes insipidus (p = 0.002), greater infection rates (p = 0.002), and fewer ventilator-free days (p = 0.001). In sTBI survivors, SAH was associated with increased lengths of stay (p < 0.001) and a higher level of care required on discharge (p = 0.004). Despite evidence that SAH is linked to poorer outcomes on univariate analyses, multivariate analysis failed to demonstrate an independent association between SAH and mortality (p = 0.969).
Conclusion
SAH was present in almost half of pediatric sTBI patients, and it was indicative of TBI severity and a higher level of care on discharge. SAH in pediatric patients was not independently associated with increased risk of mortality.
Similar content being viewed by others
Introduction
Severe traumatic brain injury (sTBI) is a major cause of pediatric morbidity and mortality [1, 2]. Head computed tomography (CT) imaging is recommended on hospital presentation after sTBI, but only a few pediatric studies have evaluated outcome prediction based on head CT abnormalities [3–7]. The most frequently reported findings on head CT after pediatric sTBI include diffuse axonal injury (DAI), edema with ventricular and/or cistern compression, midline shift, subdural hematoma, and intraparenchymal hemorrhage [3, 4, 8–10].
Subarachnoid hemorrhage (SAH) on head CT is less frequently reported after sTBI; however, it may be an important element in determining pediatric sTBI prognosis. The reported SAH incidence in adult sTBI is 33–60 % [11]. The causes of SAH in sTBI include tearing, stretching, and laceration of subarachnoid blood vessels [2, 12]. Cerebral vasospasm, ischemia, hypoxia, edema, seizures, and hydrocephalus are all sequelae of SAH [11]. The risk of cerebral vasospasm is increased by SAH, and in adult, sTBI patients are associated with ischemic injury [11, 13–16]. In contrast, a link between traumatic SAH and cerebral vasospasm in children is lacking [17, 18]. It is also unclear whether SAH is independently associated with poor outcome after sTBI or if the presence of SAH simply reflects TBI severity [11, 19].
To our knowledge, SAH has not been specifically investigated in pediatric sTBI. SAH has been reported in a wider prognostic sTBI study [8] or in mixed pediatric and adult sTBI populations [20, 21]. Clarification of SAH as a potential outcome predictor in pediatric sTBI may aid clinical sTBI management and, if appropriate, limitations of care. Based on the paucity of data surrounding SAH in pediatric sTBI, the aim of this study was to determine SAH incidence, associated factors, and its relationship to short-term outcome in pediatric sTBI patients.
Materials and Methods
The Health Sciences Research Ethics Board at Western University approved this study. We retrospectively analyzed sTBI patient data obtained from the prospectively collected written and electronic admission records of the pediatric intensive care unit (PICU), collated with the data from the provincially mandated London Health Sciences Centre (LHSC) Trauma Registry [22–25]. We first screened all pediatric trauma patients (<18 years) over a 12-year period (January 2000–December 2011) with an Injury Severity Score (ISS) ≥12. Patients suffering a sTBI were then identified by a pre-sedation GCS ≤8 and a head Maximum Abbreviated Injury Scale (MAIS) ≥4. Our sTBI population included patients with and without multi-system trauma.
All sTBI patients were admitted to the PICU located in the Children’s Hospital at LHSC, which is a regional Pediatric Level I trauma centre for South Western Ontario. Our Children’s Hospital serves a geographic area of 19,000 square kilometers with a pediatric population of over 500,000. Patients who were admitted more than 12 h after their injury were excluded.
The patient data collected included demographics, injury data, neuroimaging studies, interventions, and outcomes. Pre-sedation GCS was determined at either the scene, referring hospital, or on arrival to LHSC trauma centre. The pupillary response and hypotension were recorded on arrival to our trauma room. Hypotension was defined as a systolic blood pressure (SBP) <70 for infants (<1 year of age), a SBP <70 + (2*age) for toddlers (1–3 years of age) and children (4–9 years of age), and a SBP <90 for children ≥10 years of age [26].
The reports of the first head CT imaging, obtained within 24 h of patient admission, were collected. Images were not re-reviewed as all were read by fellowship-trained neuro-radiologists, and the inter-observer variability for radiologists interpreting TBI on head CT is considered low [27]. Specific abnormalities documented included skull fractures, cerebral edema (focal and diffuse), DAI, SAH, subdural hemorrhage (SDH), intracranial hemorrhage (ICH), brain herniation, midline shift, cerebral contusion, and ischemia.
Interventions recorded included placement of an ICP monitor, treatment with mannitol and/or 3 % hypertonic saline, induction of thiopental coma, and decompressive craniectomy. Controlled hypothermia was administered in a subset of sTBI patients as part of a larger randomized-controlled trial [28]. Additional interventions included transfusion of packed red blood cells (PRBC) or other blood products, and treatment with desamino-8-d-arginine (DDAVP) and/or vasopressin [22].
Over the study period, patients with sTBI in our institution were placed on a temperature-modulating blanket in supine position, with the head elevated to 30°. Normothermia was targeted in those sTBI patients not enrolled in hypothermia trial [28] with administration of antipyretics as needed, passive cooling and/or use of the temperature-modulating blanket. Antibiotics were not routinely administered, except for the inconsistent prophylactic use of cefazolin after ICP monitor placement [29]. Adequate analgesia and sedation were obtained with opioid and benzodiazepine infusions, respectively.
Raised ICP was generally managed as per published protocol [30]. If the ICP >20–25 mmHg for >5 min or for a rapidly raising ICP: (1) drain cerebrospinal fluid for 5 min if external ventricular drain in situ [29]; (2) mannitol 0.5 g/kg intravenously over 20 min every 6 h as needed; (3) 3 % NaCl given in boluses of 1–2 mL/kg over 5 min every 12 h as needed (first dose 2–4 mL/kg); (4) hyperventilation to a PaCO2 35 mmHg; (5) barbiturate infusion titrated to ICP and CPP; and (6) neurosurgical consult for potential decompressive craniectomy. Mannitol and 3 % NaCl were held if the measured osmolality was greater than 320 and 360 mOsm/L, respectively. Low cerebral perfusion pressure (CPP) secondary to arterial hypotension and without raised ICP was managed as follows: 0.9 % NaCl (or colloid) 10 mL/kg IV over 5–30 min as needed, followed by administration of inotropes/vasopressors.
Severe TBI outcomes in those with and without a SAH included in-hospital mortality, PICU and hospital lengths of stays (LOS), ventilator-free days (unventilated days in the first 28 days of admission), and discharge destination. Discharge destinations included chronic rehabilitation hospital, acute care hospital, or home. Discharge to a chronic rehabilitation hospital is a less favorable outcome, as it indicates that the patient was less functionally independent on discharge.
Acute central diabetes insipidus (CDI) was defined as polyuria (urine output >4 mL/kg/h for children <70 kg; >300 mL/h for adult size children ≥70 kg) for at least 2 consecutive hours, hypernatremia (serum Na >145 mmol/L), high serum osmolality (>300 mOsm/kg), and low urine osmolality (<300 mOsm/kg) at the time of diagnosis [22]. Confirmation of CDI was based on symptom reversal with administration of DDAVP and/or vasopressin.
Infections in sTBI patients were described previously [23]. Culture positive urinary tract infections (single organism; ≥105 colony forming units/mL) were determined within the first 48 h of admission. Nosocomial infections recorded were identified by Centers for Disease Control criteria and included ventilator associated pneumonia (http://www.cdc.gov/hai/vap/vap.html), catheter-associated urinary tract infection (http://www.cdc.gov/HAI/ca_uti/uti.html), and central line blood stream infection (http://www.cdc.gov/hai/bsi/bsi.html). Meningitis and wound infections were confirmed by positive cultures of a single organism.
Continuous variables were found to deviate considerably from normality and, hence, medians with interquartile ranges (IQR) were presented. Between-group comparisons were made using the Mann–Whitney U test. For categorical variables, the frequencies and percentages were presented, and between-group comparisons were made using the Pearson’s Chi square or Fisher’s exact test where appropriate. PICU and hospital LOS and post-discharge destination were assessed for survivors only. For all analyses, a p value of <0.05 was considered statistically significant.
Multivariable logistic regression modeling was performed with SAH as the outcome variable. Variables identified a priori to be assessed for inclusion in the model were age, gender, ISS, motor vehicle collision, laboratory results (INR, PTT, and platelets), and associated CT findings (cerebral contusion, edema, DAI, IVH, SDH, EDH, midline shift, herniation, and ischemia). Variables found to be significant in univariate analyses at the 0.25 level were then entered and allowed to be removed from the model at the 0.10 level in a backward elimination strategy. Sensitivity analyses were performed using different modeling methods (i.e., forward stepwise logistic regression) [31]. For the forward stepwise method, variables were also entered at the 0.25 level and removed at the 0.10 level.
A second multivariable logistic regression analysis was undertaken to determine the association of patient’s demographics, physiologic variables, neuroimaging abnormalities, including SAH, and overall injury severity on mortality, while controlling for the possible confounding effects of these variables on the relationships. Possible confounders were identified a priori. Variables considered included age [assessed both as a continuous variable and dichotomized into adolescent (age 13–17 years) or non-adolescent (age ≤12 years)], gender, ISS, hypotension on arrival to hospital, pre-sedation motor GCS, fixed pupillary response on hospital admission, SAH, and all other types of TBI on CT findings. A backward elimination procedure was utilized in which variables considered a priori to be clinically important (i.e., ISS, SAH) were forced into the model. Other variables found to be significant in univariate analyses at the 0.25 level were then entered and allowed to be removed from the model at the 0.10 level. Sensitivity analyses were performed using different modeling methods (i.e., forward stepwise logistic regression) [31].
For both models, the Hosmer–Lemeshow statistic was calculated to evaluate model fit, and the C statistic was calculated to evaluate the predictive accuracy of our logistic regression model. Multi-collinearity was assessed with a correlation coefficient analysis, and variables were removed with strong collinearity. The final models were used to determine the estimated odds (OR) of SAH and mortality, respectively, for each variable, adjusted for confounders. All analyses were performed using IBM® SPSS® Statistics Version 21 (IBM Corporation, Armonk, NY).
Results
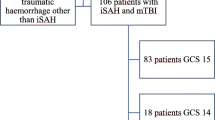
A total of 818 pediatric severe trauma patients were screened, with 180 patients meeting the sTBI study inclusion criteria (GCS ≤8 and MAIS ≥4). Of those 180 sTBI patients, 171 had an admission head CT available for review. SAH was found in 42 % (n = 71/171) of sTBI patients.
The admission head CTs were compared for additional abnormalities in those sTBI patients with and without SAH (Table 1). Patients who had SAH on initial head CT were also found to have a greater number of imaging signs suggestive of worse injury, including skull fractures (p < 0.001), cerebral edema (p = 0.015), contusion (p = 0.007), DAI (p < 0.001), and IVH (p < 0.001).
When comparing the admission demographics and injury variables between sTBI patients with and without SAH, there were no differences between the age, sex, and weight of the patients (Table 2). A total of 20 patients were abused/assaulted, with 18 patient cases defined as shaken baby. There were also no differences in etiologies between sTBI patients with and without SAH. The only significant findings between groups were higher ISS (p = 0.032) and more frequent fixed pupil(s) on admission (p = 0.001) in the sTBI patients with SAH (Table 2) .
A comparison of admission laboratory values in patients with and without SAH (Table 3) showed that both partial thromboplastin time (PTT) and international normalized ratio (INR) were significantly higher in SAH patients (p = 0.003 and p = 0.017, respectively), with platelets significantly lower with a median of 153 in the SAH group (vs. 210.5; p = 0.005). The other laboratory values did not differ between the SAH and no SAH groups (Table 3).
Severe TBI patients with SAH were more likely to receive an ICP monitor and ICP lowering therapies including mannitol, hypertonic saline, decompressive craniectomy, and thiopental coma (p < 0.05; Table 4). Additionally, SAH patients received more transfusions, including both PRBC and other blood products (p < 0.05). Administration of DDAVP/vasopressin, as treatment for acute CDI, was also used more frequently in those sTBI patients with SAH (p = 0.002). Patients enrolled in the therapeutic hypothermia trial were randomized similarly between sTBI patients with and without SAH (Table 4).
The outcomes for sTBI patients with and without SAH are presented in Table 5. Patients with SAH had increased rates of mortality, acute CDI, and infection (p < 0.01). Fewer ventilator-free days were documented in sTBI patients with SAH (p = 0.001). For sTBI survivors, those with SAH had significantly greater PICU and hospital LOS (p < 0.001; Table 5). Patients with SAH were less likely to be discharged home and more likely to be sent to a chronic rehabilitation center (p = 0.004).
The first multivariate logistic regression modeling analysis was conducted to examine the association of patient and injury variables with SAH in this group of sTBI patients (Table 6). Our sensitivity analysis revealed the two nearly identical models with both forward stepwise and backward elimination, with no multi-collinearity of the variables evident in either model. The strength of association for all variables was very similar in both models. The only difference in the models was SDH that was statistically associated with SAH in the forward entry regression (p = 0.049 vs. 0.060). This model was selected as it had slightly improved model performance in classifying outcome. For the model performance evaluation, a test of the full model against a constant only model was statistically significant, indicating that the predictor variables reliably distinguished between patients with or without SAH (Omnibus test of model coefficients, X 25 = 41.43, p < 0.001). A good fit of the model was achieved (Hosmer and Lemeshow X 27 = 9.17, p = 0.241) with overall classification success of mortality at 73 % for the final model. The C statistic was 0.780, indicating fair predictive accuracy of our model. The Wald criterion demonstrated that SAH was significantly associated with fixed pupillary response (OR 2.48, p = 0.020), cerebral contusion (OR 3.32, p = 0.002), DAI (OR 3.56, p = 0.003), IVH (OR 3.08, p = 0.010), and SDH (OR 2.09; p = 0.049).
The unadjusted OR of SAH on mortality was 2.46 (p = 0.010). The results of the second multivariable logistic regression are presented in Table 7. Review of the correlation coefficient matrix did not reveal any multi-collinearity between the variables in the final model. The same final model was achieved with a forward stepwise procedure as with backward elimination. There was no evidence of a lack of fit (Hosmer and Lemeshow X 28 = 2.59, p = 0.958), with an overall classification success of mortality at 94 % for the final model. The C statistic was 0.989, indicating excellent predictive accuracy of our model. CDI (OR 282.42, p < 0.001), fixed pupillary response (OR 34.51, p = 0.001), pre-sedation motor GCS (OR 0.18, p = 0.006), ischemia (OR 28.69, p = 0.006), and hypotension upon hospital arrival (OR 11.91, p = 0.027) were found to be significantly associated with mortality. However, after adjusting for other characteristics in the model, there was no evidence of a difference in mortality between those with and without SAH (OR 1.04, p = 0.969).
Discussion
We investigated SAH incidence, associated factors, and its relationship to outcome in pediatric sTBI patients. Our data showed that SAH was a frequent finding in 42 % sTBI patients, and while reflective of TBI severity failed to be independently associated with mortality. To our knowledge, this study is the first to specifically analyze SAH in pediatric sTBI.
Head CT imaging is critical in the acute evaluation of sTBI, and identified abnormalities have been used previously to predict outcome in both pediatrics [3–5] and adults [20, 32]. Multiple adult studies have demonstrated SAH to be a prognosticator of poor outcome after sTBI [19, 33–35]; however, only one pediatric study had examined this relationship [8]. SAH was shown to be an independent predictor of mortality in a large cohort of 753 adult sTBI patients [32]. Furthermore, adding SAH to the prognostic performance of the Marshall CT classification was shown to further assist in predicting outcome in moderate to severe TBI patients aged 15–65 [20]. While the IMPACT (Independent Mission for Prognosis and Analysis of Clinical Trials in sTBI) database indicated that age was the single most important predictive factor, followed by GCS motor score and pupil reactivity, addition of the Marshall CT classification, and SAH provided further independent predictive information [21]. The CRASH (Corticosteroid Randomization After Severe Head Injury) study investigated 10,008 adult patients and found SAH to be an independent prognostic indicator, and included SAH in there prognostic online tool with GCS, age, pupil reactivity, major extra cranial injury, and four additional CT findings [36].
Prognostic studies for pediatric sTBI are generally lacking [9, 10, 37], with some studies focusing specifically on head CT imaging [3, 4]. The use of neuroimaging in predicting poor outcome of pediatric sTBI patients has included the Marshall CT classification, which does not incorporate SAH [3]. To our knowledge, only one pediatric sTBI study included SAH, together with other clinical factors, to predict poor functioning on a dichotomized Glasgow Outcome Scale [8]. This latter study failed to find an independent association between SAH and functional outcome after sTBI but suffered from a limited patient number relative to the number of variables investigated.
Laboratory values are infrequently included in sTBI prognostic evaluation; however, they have been shown to assist in determining patient outcome [21, 24, 38, 39]. Traumatic brain injury has been shown to increase risk of coagulation abnormalities [39–44], which can lead to progressive hemorrhagic injury [45, 46] and poorer outcomes [39, 41, 44]. Our data showed that coagulation factors including PTT, INR, and platelets were significantly worse in the sTBI patients with SAH. However, upon regression analysis, the coagulation variables were not independently associated with SAH. SAH was independently associated with fixed pupils, contusion, DAI, IVH, and SDH.
Adult studies have generally demonstrated an independent association between SAH with TBI outcomes [19, 33, 34]. One study investigated the clinical significance of SAH on initial head CT in 819 TBI patients and found SAH to be correlated with worse outcome [33]. Others investigated 750 adult patients from the European Brain Injury Consortium and found SAH to be independently associated with lower GCS and increased mortality [19]. This latter study also reported that the distribution of SAH on head CT was not associated with outcome.
Our data suggested that the presence of SAH reflects worse TBI, but it is not independently associated with mortality. Increased TBI severity in the SAH cohort is illustrated by the increased number of patients with fixed pupils, the greater number of abnormalities found on initial head CT and the larger number of interventions provided. Additionally, sTBI patients with SAH also suffered increased complications (infections and CDI), had a greater LOS, and had a worse disposition on discharge. Despite a greater percentage of deaths in the SAH cohort, SAH was not independently associated with mortality. Similar to previous studies, mortality was independently associated with motor GCS, absent pupillary reflex, hypotension, and ischemia on initial head CT [8, 9, 21, 24, 25].
In our study, sTBI patients with SAH were more likely to develop acute CDI [22]. The presence of SAH has been reported to be associated with pituitary dysfunction [47]. In a population of adults who required neurocritical care for CDI, 41 % of cases were secondary to SAH and in those with sTBI, SAH was associated with impending brain death and mortality [48]. Although traumatic SAH and acute CDI may be associated merely because of their shared relationship with more severe brain injury, SAH-induced vasospasm may place patients at higher risk of CDI through neurohypophyseal ischemic injury [49].
There is no clear consensus on the pathophysiology of SAH in sTBI. Adult studies have shown that SAH results in cerebral vasospasm leading to ischemia [14–16, 50], versus SAH being solely a marker of increase TBI severity [11, 19, 51]. Vasospasm has been shown to occur in pediatric sTBI patients in one study; however, the vasospasm was not shown to be specifically secondary to SAH [18]. The relationship between SAH and vasospasm in children remains unknown.
Our study has limitations. First, ours was a retrospective single center study. Nonetheless, we believe our data to be relevant for other Level 1 trauma centers, as we used strict inclusion criteria for sTBI. Second, there was a risk of missed data; however, we minimized this possibility by using our provincially mandated, quality-controlled trauma registry to capture most data points. Third, we did not differentiate either the anatomical location of SAH [35], or the maximal thickness of subarachnoid blood [52]. Using a SAH, CT grading scale could help predict outcome at time of discharge but would require a greater number of sTBI patients to investigate [35, 53]. Fourth, our study cannot control for clinician bias and whether or not they were more likely to treat a patient based on initial head CT findings. Finally, we used mortality as the primary outcome and discharge destination as a surrogate for functional outcome in survivors. Future studies should attempt to include larger patient populations with functional outcome scoring (i.e., Glasgow Outcome Score and/or Pediatric Cerebral Performance Category) at defined times (i.e., 6 months post-discharge).
Conclusion
We report that SAH was a frequent finding after pediatric sTBI, occurring in almost half of the patients. SAH was associated with worse sTBI short-term outcomes on univariate analyses, but it was not independently associated with mortality on multivariate analysis. The presence of SAH may be predictive of outcomes in sTBI survivors, including longer recovery times and worse functional outcomes. Future prospective investigations should utilize an SAH grading system in a larger cohort of pediatric sTBI patients and incorporate functional outcome scoring at defined periods. In addition, the potential relationship between SAH and cerebral vasospasm should be delineated.
References
Sigurta A, Zanaboni C, Canavesi K, Citerio G, Beretta L, Stocchetti N. Intensive care for pediatric traumatic brain injury. Intensiv Care Med. 2013;39:129–36.
Morrison G, Fraser DD, Cepinskas G. Mechanisms and consequences of acquired brain injury during development. Pathophysiology. 2013;20:49–57.
Claret Teruel G, Palomeque Rico A, Cambra Lasaosa FJ, Catala Temprano A, Noguera Julian A, Costa Clara JM. Severe head injury among children: computed tomography evaluation as a prognostic factor. J Pediatr Surg. 2007;42:1903–6.
Tomberg T, Rink U, Pikkoja E, Tikk A. Computerized tomography and prognosis in paediatric head injury. Acta Neurochir (Wien). 1996;138:543–8.
Ong L, Selladurai BM, Dhillon MK, Atan M, Lye MS. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr Neurosurg. 1996;24:285–91.
Levin HS, Aldrich EF, Saydjari C, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery 1992;31:435–43; discussion 43–4.
Hirsch W, Schobess A, Eichler G, Zumkeller W, Teichler H, Schluter A. Severe head trauma in children: cranial computer tomography and clinical consequences. Paediatr Anaesth. 2002;12:337–44.
Pillai S, Praharaj SS, Mohanty A, Kolluri VR. Prognostic factors in children with severe diffuse brain injuries: a study of 74 patients. Pediatr Neurosurg. 2001;34:98–103.
Chiaretti A, Piastra M, Pulitano S, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst. 2002;18:129–36.
Feickert H-J, Drommer S, Heyer R. Severe head injury in children: impact of risk factors on outcome. J Trauma. 1999;47:33–8.
Armin SS, Colohan AR, Zhang JH. Traumatic subarachnoid hemorrhage: our current understanding and its evolution over the past half century. Neurol Res. 2006;28:445–52.
Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9.
Taneda M, Kataoka K, Akai F, Asai T, Sakata I. Traumatic subarachnoid hemorrhage as a predictable indicator of delayed ischemic symptoms. J Neurosurg. 1996;84:762–8.
Martin NA, Doberstein C, Zane C, Caron MJ, Thomas K, Becker DP. Posttraumatic cerebral arterial spasm: transcranial Doppler ultrasound, cerebral blood flow, and angiographic findings. J Neurosurg. 1992;77:575–83.
Compton JS, Teddy PJ. Cerebral arterial vasospasm following severe head injury: a transcranial Doppler study. Br J Neurosurg. 1987;1:435–9.
Oertel M, Boscardin WJ, Obrist WD, et al. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg. 2005;103:812–24.
Shahlaie K, Keachie K, Hutchins IM, et al. Risk factors for posttraumatic vasospasm. J Neurosurg. 2011;115:602–11.
O’Brien NF, Reuter-Rice KE, Khanna S, Peterson BM, Quinto KB. Vasospasm in children with traumatic brain injury. Intensiv Care Med. 2010;36:680–7.
Servadei F, Murray GD, Teasdale GM, et al. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery 2002;50:261–7; discussion 7–9.
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005;57:1173–82; discussion 82.
Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–37.
Alharfi IM, Stewart TC, Foster J, Morrison GC, Fraser DD. Central diabetes insipidus in pediatric severe traumatic brain injury. Pediatr Crit Care Med. 2013;14:203–9.
Alharfi IM, Stewart TC, Helali IA, Daoud H, Fraser DD. Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J Neurotrauma. 2014;31:452–8.
Alharfi IM, Stewart TC, Kelly SH, Morrison GC, Fraser DD. Hypernatremia is associated with increased risk of mortality in pediatric severe traumatic brain injury. J Neurotrauma. 2013;30:361–6.
Stewart TC, Alharfi IM, Fraser DD. The role of serious concomitant injuries in the treatment and outcome of pediatric severe traumatic brain injury. J Trauma Acute Care Surg. 2013;75:836–42.
American College of Surgeons. Committe on Trauma. Advanced trauma life support program for doctors : ATLS student course manual. 8th ed. Chicago: American College of Surgeons; 2008.
Chun KA, Manley GT, Stiver SI, et al. Interobserver variability in the assessment of CT imaging features of traumatic brain injury. J Neurotrauma. 2010;27:325–30.
Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–56.
Ngo QN, Ranger A, Singh RN, Kornecki A, Seabrook JA, Fraser DD. External ventricular drains in pediatric patients. Pediatr Crit Care Med. 2009;10:346–51.
Hutchison J, Ward R, Lacroix J, et al. Hypothermia pediatric head injury trial: the value of a pretrial clinical evaluation phase. Dev Neurosci. 2006;28:291–301.
Hosmer D, Lemeshow S. Applied logistic regression. New York: Wiley; 1989.
Eisenberg HM, Gary HE Jr, Aldrich EF, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–98.
Kakarieka A, Braakman R, Schakel EH. Clinical significance of the finding of subarachnoid blood on CT scan after head injury. Acta Neurochir (Wien). 1994;129:1–5.
Greene KA, Jacobowitz R, Marciano FF, Johnson BA, Spetzler RF, Harrington TR. Impact of traumatic subarachnoid hemorrhage on outcome in nonpenetrating head injury. Part II: relationship to clinical course and outcome variables during acute hospitalization. J Trauma. 1996;41:964–71.
Greene KA, Marciano FF, Johnson BA, Jacobowitz R, Spetzler RF, Harrington TR. Impact of traumatic subarachnoid hemorrhage on outcome in nonpenetrating head injury. Part I: a proposed computerized tomography grading scale. J Neurosurg. 1995;83:445–52.
Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–9.
Kalff R, Kocks W, Pospiech J, Grote W. Clinical outcome after head injury in children. Childs Nerv Syst. 1989;5:156–9.
Van Beek JG, Mushkudiani NA, Steyerberg EW, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:315–28.
Franschman G, Boer C, Andriessen TM, et al. Multicenter evaluation of the course of coagulopathy in patients with isolated traumatic brain injury: relation to CT characteristics and outcome. J Neurotrauma. 2012;29:128–36.
Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit Care. 2004;1:479–88.
Chiaretti A, Pezzotti P, Mestrovic J, et al. The influence of hemocoagulative disorders on the outcome of children with head injury. Pediatr Neurosurg. 2001;34:131–7.
Lustenberger T, Talving P, Kobayashi L, et al. Time course of coagulopathy in isolated severe traumatic brain injury. Injury. 2010;41:924–8.
Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir 2008;150:165–75; discussion 75.
Greuters S, van den Berg A, Franschman G, et al. Acute and delayed mild coagulopathy are related to outcome in patients with isolated traumatic brain injury. Crit Care. 2011;15:R2.
Oertel M, Kelly DF, McArthur D, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109–16.
Engstrom M, Romner B, Schalen W, Reinstrup P. Thrombocytopenia predicts progressive hemorrhage after head trauma. J Neurotrauma. 2005;22:291–6.
Aimaretti G, Ambrosio MR, Di Somma C, et al. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf). 2004;61:320–6.
Wong MF, Chin NM, Lew TW. Diabetes insipidus in neurosurgical patients. Ann Acad Med Singap. 1998;27:340–3.
Aminmansour B, Ghorbani A, Sharifi D, Shemshaki H, Ahmadi A. Cerebral vasospasm following traumatic subarachnoid hemorrhage. J Res Med Sci. 2009;14:343–8.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9.
Mattioli C, Beretta L, Gerevini S, et al. Traumatic subarachnoid hemorrhage on the computerized tomography scan obtained at admission: a multicenter assessment of the accuracy of diagnosis and the potential impact on patient outcome. J Neurosurg. 2003;98:37–42.
Tu CJ, Liu JS, Song DG, et al. Maximum thickness of subarachnoid blood is associated with mortality in patients with traumatic subarachnoid haemorrhage. J Int Med Res. 2011;39:1757–65.
Chieregato A, Fainardi E, Morselli-Labate AM, et al. Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery 2005;56:671–80; discussion 80.
Acknowledgment
We thank “LW Stitt Statistical Services” for independent methodological and statistical review.
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hochstadter, E., Stewart, T.C., Alharfi, I.M. et al. Subarachnoid Hemorrhage Prevalence and Its Association with Short-Term Outcome in Pediatric Severe Traumatic Brain Injury. Neurocrit Care 21, 505–513 (2014). https://doi.org/10.1007/s12028-014-9986-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-9986-7