Abstract
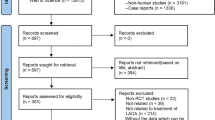
We performed a systematic review and meta-analysis to assess the efficacy and safety of antigen-based immunotherapies in tertiary prevention of autoimmune diabetes. We searched for randomised controlled trials testing antigen-based immunotherapies in patients with recent-onset type 1 diabetes or latent autoimmune diabetes of adults in MEDLINE, COCHRANE and EMBASE databases, trial registries, conference proceedings and reference lists of pertinent records. Primary outcomes were fasting and stimulated C-peptide (after glucagon or mixed meal stimulation). Change in glycosylated haemoglobin (HbA1c), daily insulin needs and incidence of any or severe hypoglycaemic events or severe adverse events were secondary outcomes. Fifteen studies were included in the meta-analysis. Overall, there was no difference in fasting [weighted mean difference (WMD) 0.01 nmol/L; 95 % confidence interval (CI) −0.09, 0.11; I 2 = 73 %] or mixed meal stimulated C-peptide (WMD 0.02 nmol/L/min; 95 % CI −0.08, 0.12; I 2 = 50 %) compared with placebo. Glucagon stimulated C-peptide was maintained higher (WMD 0.13 nmol/L/min; 95 % CI 0.05, 0.21; I 2 = 0 %) in patients treated with Diapep277. Moreover, there was no change in daily insulin needs (WMD 0.02 IU/kg; 95 % CI −0.04, 0.09; I 2 = 51 %) or HbA1c (WMD −0.06 %; 95 % CI −0.35, 0.23; I 2 = 42 %) vs. placebo. Finally, there was no effect on the incidence of severe hypoglycaemic events or overall serious adverse events [risk ratio 0.94, 95 % CI 0.62, 1.41; I 2 = 0 % and 0.87; 95 % CI 0.53, 1.44; I 2 = 0 %, respectively). Antigen-based immunotherapies are not effective in preventing the progression of autoimmune diabetes in newly diagnosed patients.





Similar content being viewed by others
References
B. Brooks-Worrell, J.P. Palmer, Prevention versus intervention of type 1 diabetes. Clin. Immunol. 149(3), 332–338 (2013)
M. Rewers, P. Gottlieb, Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future. Diabetes Care 32(10), 1769–1782 (2009)
S. Robert, H. Korf, C. Gysemans, C. Mathieu, Antigen-based vs. systemic immunomodulation in type 1 diabetes: the pros and cons. Islets 5(2), 53–66 (2013)
L. Chaillous, H. Lefevre, C. Thivolet, C. Boitard, N. Lahlou, C. Atlan-Gepner, B. Bouhanick, A. Mogenet, M. Nicolino, J.C. Carel, P. Lecomte, R. Marechaud, P. Bougneres, B. Charbonnel, P. Sai, Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Lancet 356(9229), 545–549 (2000)
P. Pozzilli, D. Pitocco, N. Visalli, M.G. Cavallo, R. Buzzetti, A. Crino, S. Spera, C. Suraci, G. Multari, M. Cervoni, M.L. Manca Bitti, M.C. Matteoli, G. Marietti, F. Ferrazzoli, M.R. Cassone Faldetta, C. Giordano, M. Sbriglia, E. Sarugeri, G. Ghirlanda, No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia 43(8), 1000–1004 (2000)
C.D. Agardh, C.M. Cilio, A. Lethagen, K. Lynch, R.D. Leslie, M. Palmer, R.A. Harris, J.A. Robertson, A. Lernmark, Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J. Diabetes Complications 19(4), 238–246 (2005)
S. Fourlanos, C. Perry, S.A. Gellert, E. Martinuzzi, R. Mallone, J. Butler, P.G. Colman, L.C. Harrison, Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 60(4), 1237–1245 (2011)
T. Orban, K. Farkas, H. Jalahej, J. Kis, A. Treszl, B. Falk, H. Reijonen, J. Wolfsdorf, A. Ricker, J.B. Matthews, N. Tchao, P. Sayre, P. Bianchine, Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J. Autoimmun. 34(4), 408–415 (2010)
I. Raz, D. Elias, A. Avron, M. Tamir, M. Metzger, I.R. Cohen, Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 358(9295), 1749–1753 (2001)
J.P. Palmer, G.A. Fleming, C.J. Greenbaum, K.C. Herold, L.D. Jansa, H. Kolb, J.M. Lachin, K.S. Polonsky, P. Pozzilli, J.S. Skyler, M.W. Steffes, C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes 53(1), 250–264 (2004)
U.S. Department of Health and Human Services Food and Drug Administration (2008) Guidance for Industry Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention. in., pp. 16–17
JPT Higgins, S. Green (ed.). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.handbook.cochrane.org. Accessed 4 July 2016
Type 1 Diabetes TrialNet Protocol Development Guidelines. in: TrialNet, T.D. (ed.). p. 8. (2011). https://www.diabetestrialnet.org/documents/protocol-dev-guiderev7-27-Secure.pdf. Accessed 4 July 2016
C.J. Greenbaum, T. Mandrup-Poulsen, P.F. McGee, T. Battelino, B. Haastert, J. Ludvigsson, P. Pozzilli, J.M. Lachin, H. Kolb, Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 31(10), 1966–1971 (2008)
J. Higgins, S. Green, in: Cochrane Handbook for Systematic Reviews of Interventions, (2008), pp. 485-488
S.P. Hozo, B. Djulbegovic, I. Hozo, Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13 (2005)
J. Higgins, S. Green, in: Cochrane Handbook for Systematic Reviews of Interventions, (2008), pp. 553-555
J. Higgins, S. Green, in: Cochrane Handbook for Systematic Reviews of Interventions, (2008), pp. 264-265
J. Higgins, S. Green, in: Cochrane Handbook for Systematic Reviews of Interventions, (2008), p. 266
T. Trikalinos, OpenMeta[Analyst]. Brown School of Public Health, http://www.cebm.brown.edu/openmeta/download.html. Accessed 4 July 2016
J.P. Higgins, D.G. Altman, P.C. Gotzsche, P. Juni, D. Moher, A.D. Oxman, J. Savovic, K.F. Schulz, L. Weeks, J.A. Sterne; Cochrane Bias Methods, G., Cochrane Statistical Methods, G., The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011)
G. Guyatt, A.D. Oxman, E.A. Akl, R. Kunz, G. Vist, J. Brozek, S. Norris, Y. Falck-Ytter, P. Glasziou, H. DeBeer, R. Jaeschke, D. Rind, J. Meerpohl, P. Dahm, H.J. Schunemann, GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64(4), 383–394 (2011)
B. Ergun-Longmire, J. Marker, A. Zeidler, R. Rapaport, P. Raskin, B. Bode, D. Schatz, A. Vargas, D. Rogers, S. Schwartz, J. Malone, J. Krischer, N.K. Maclaren, Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann. N. Y. Acad. Sci. (2004). doi:10.1196/annals.1309.057
D.G. Alleva, R.A. Maki, A.L. Putnam, J.M. Robinson, M.S. Kipnes, P. Dandona, J.B. Marks, D.L. Simmons, C.J. Greenbaum, R.G. Jimenez, P.J. Conlon, P.A. Gottlieb, Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand. J. Immunol. 63(1), 59–69 (2006)
V.A. Huurman, K. Decochez, C. Mathieu, I.R. Cohen, B.O. Roep, Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab. Res. Rev. 23(4), 269–275 (2007)
L. Lazar, R. Ofan, N. Weintrob, A. Avron, M. Tamir, D. Elias, M. Phillip, Z. Josefsberg, Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab. Res. Rev. 23(4), 286–291 (2007)
N.C. Schloot, G. Meierhoff, C. Lengyel, G. Vándorfi, J. Takács, P. Pánczél, L. Barkai, L. Madácsy, T. Oroszlán, P. Kovács, G. Sütö, T. Battelino, N. Hosszufalusi, G. Jermendy, Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab. Res. Rev. 23(4), 276–285 (2007)
J. Ludvigsson, M. Faresjö, M. Hjorth, S. Axelsson, M. Chéramy, M. Pihl, O. Vaarala, G. Forsander, S. Ivarsson, C. Johansson, A. Lindh, N.O. Nilsson, J. Aman, E. Ortqvist, P. Zerhouni, R. Casas, GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. (2008). doi:10.1056/NEJMoa0804328
M. Walter, A. Philotheou, F. Bonnici, A.G. Ziegler, R. Jimenez, No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care 32(11), 2036–2040 (2009)
D.K. Wherrett, B. Bundy, D.J. Becker, L.A. DiMeglio, S.E. Gitelman, R. Goland, P.A. Gottlieb, C.J. Greenbaum, K.C. Herold, J.B. Marks, R. Monzavi, A. Moran, T. Orban, J.P. Palmer, P. Raskin, H. Rodriguez, D. Schatz, D.M. Wilson, J.P. Krischer, J.S. Skyler, Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378(9788), 319–327 (2011)
J. Ludvigsson, D. Krisky, R. Casas, T. Battelino, L. Castano, J. Greening, O. Kordonouri, T. Otonkoski, P. Pozzilli, J.J. Robert, H.J. Veeze, J. Palmer, U. Samuelsson, H. Elding Larsson, J. Aman, G. Kardell, J. Neiderud Helsingborg, G. Lundstrom, E. Albinsson, A. Carlsson, M. Nordvall, H. Fors, C.G. Arvidsson, S. Edvardson, R. Hanas, K. Larsson, B. Rathsman, H. Forsgren, H. Desaix, G. Forsander, N.O. Nilsson, C.G. Akesson, P. Keskinen, R. Veijola, T. Talvitie, K. Raile, T. Kapellen, W. Burger, A. Neu, I. Engelsberger, B. Heidtmann, S. Bechtold, D. Leslie, F. Chiarelli, A. Cicognani, G. Chiumello, F. Cerutti, G.V. Zuccotti, A. Gomez Gila, I. Rica, R. Barrio, M. Clemente, M.J. Lopez Garcia, M. Rodriguez, I. Gonzalez, J.P. Lopez, M. Oyarzabal, H.M. Reeser, R. Nuboer, P. Stouthart, N. Bratina, N. Bratanic, M. de Kerdanet, J. Weill, N. Ser, P. Barat, A.M. Bertrand, J.C. Carel, R. Reynaud, R. Coutant, S. Baron, GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366(5), 433–442 (2012)
I. Raz, A.G. Ziegler, T. Linn, G. Schernthaner, F. Bonnici, L.A. Distiller, C. Giordano, F. Giorgino, L. de Vries, D. Mauricio, V. Prochazka, J. Wainstein, D. Elias, A. Avron, M. Tamir, R. Eren, D. Peled, S. Dagan, I.R. Cohen, P. Pozzilli, Treatment of recent-onset type 1 diabetic patients with DiaPep277: results of a double-blind, placebo-controlled, randomized phase 3 trial. Diabetes Care 37(5), 1392–1400 (2014)
J. Ludvigsson, M. Hjorth, M. Cheramy, S. Axelsson, M. Pihl, G. Forsander, N.O. Nilsson, B.O. Samuelsson, T. Wood, J. Aman, E. Ortqvist, R. Casas, Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: a randomised controlled trial. Diabetologia 54(3), 634–640 (2011)
DIAPREVENT - A Phase III Study to Investigate the Impact of Diamyd in Patients Newly Diagnosed With Type 1 Diabetes (USA). https://clinicaltrials.gov/ct2/show/study/NCT00751842?term=NCT00751842&rank=1. Accessed 17 March 2016
DIABGAD - Trial to Preserve Insulin Secretion in Type 1 Diabetes Using GAD-Alum (Diamyd) in Combination With Vitamin D and Ibuprofen. https://clinicaltrials.gov/ct2/show/NCT01785108?term=NCT01785108&rank=1. Accessed 17 March 2016
Diabetes Virus Detection Project, Intervention With GAD-alum (DiViD). https://clinicaltrials.gov/ct2/show/NCT01129232?term=NCT01129232&rank=1. Accessed 17 March 2016
Safety, Tolerability, Immunological and Clinical Efficacy of Multiple Subcutaneous Doses of DiaPep277 in Latent Autoimmune Diabetes in Adults (LADA). https://clinicaltrials.gov/ct2/show/NCT00058981?term=NCT00058981&rank=1. Accessed 17 March 2016
Efficacy and Safety Study of DiaPep277 in Newly Diagnosed Type 1 Diabetes Adults (DIA-AID2). https://clinicaltrials.gov/ct2/show/NCT01103284?term=NCT01103284&rank=1. Accessed 17 March 2016
C.D. Agardh, K. Lynch, J.A. Robertson, Safety of GAD65 Immunomodulation. During One Year Follow-Up in GAD65 Autoantibody Positive Type 2 Diabetes Patients. Diabetes 56. Suppl. 1, 1240-P. (2007)
C.D. Agardh, K.F. Lynch, M. Palmer, K. Link, A. Lernmark, GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 52(7), 1363–1368 (2009)
D. Elias, A. Avron, M. Tamir, I. Raz, DiaPep277 preserves endogenous insulin production by immunomodulation in type 1 diabetes. Ann. N. Y. Acad. Sci. 1079, 340–344 (2006)
I. Raz, A.G. Ziegler, T. Linn, G. Schernthaner, F. Bonnici, L.A. Distiller, C. Giordano, F. Giorgino, L. de Vries, D. Mauricio, V. Prochazka, J. Wainstein, D. Elias, A. Avron, M. Tamir, R. Eren, D. Peled, S. Dagan, I.R. Cohen, P. Pozzilli, Treatment of recent-onset type 1 diabetic patients with DiaPep277: results of a double-blind, placebo-controlled, randomized phase 3 trial. Diabetes Care 37, 1392–1400 (2014). doi:10.2337/dc13-1391 Diabetes Care 38(1), 178 (2015)
G.Y. Gandhi, M.H. Murad, D.N. Flynn, M.B. Elamin, P.J. Erwin, V.M. Montori, Y.C. Kudva, Immunotherapeutic agents in type 1 diabetes: a systematic review and meta-analysis of randomized trials. Clin. Endocrinol. 69(2), 244–252 (2008)
J. Ludvigsson, M. Cheramy, S. Axelsson, M. Pihl, L. Akerman, R. Casas, GAD-treatment of children and adolescents with recent-onset type 1 diabetes preserves residual insulin secretion after 30 months. Diabetes Metab. Res. Rev. 30(5), 405–414 (2014)
M. Cokol, F. Ozbay, R. Rodriguez-Esteban, Retraction rates are on the rise. EMBO Rep. 9(1), 2 (2008)
F.C. Fang, R.G. Steen, A. Casadevall, Misconduct accounts for the majority of retracted scientific publications. Proc. Natl. Acad. Sci. USA 109(42), 17028–17033 (2012)
R.G. Steen, Retractions in the medical literature: how can patients be protected from risk? J. Med. Ethics 38(4), 228–232 (2012)
P. Perel, I. Roberts, Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst. Rev. Cd000567 (2011)
Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. in: CHMP (ed.). European Medicines Agency (2012). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed 4 July 2016
C.A. Beam, S.E. Gitelman, J.P. Palmer, Recommendations for the definition of clinical responder in insulin preservation studies. Diabetes 63(9), 3120–3127 (2014)
Acknowledgments
CR and AT conceived the study. CR, AGT and AT designed the study. CR and EB searched the scientific literature. CR and MR did data extraction. CR and MS did risk of bias assessment and grading of recommendations. CR and AL conducted the statistical analysis and ABH contributed to the analysis. CR, AL and AT drafted the paper. EB, MR and AGT contributed to the interpretation of the findings. All authors edited and approved the final version of the manuscript to be published. AT had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rizava, C., Bekiari, E., Liakos, A. et al. Antigen-based immunotherapies do not prevent progression of recent-onset autoimmune diabetes: a systematic review and meta-analysis. Endocrine 54, 620–633 (2016). https://doi.org/10.1007/s12020-016-1033-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-1033-3