Abstract
Current protocols for inducing osteogenic differentiation in mesenchymal stem/stromal cells (MSCs) in culture for tissue engineering applications depend on the use of biochemical supplements. However, standard in vitro culture conditions expose cells to ambient oxygen concentrations and high levels of serum (21 % O2, 10 % FBS) that do not accurately recapitulate the physiological milieu. While we and others have examined MSC behavior under hypoxia, the synergistic effect of low serum levels, such as those present in ischemic injury sites, on osteogenic differentiation has not been clearly examined. We hypothesized that a concomitant reduction of serum and O2 would enhance in vitro osteogenic differentiation of MSCs by more accurately mimicking the fracture microenvironment. We show that serum deprivation, in conjunction with hypoxia, potentiates osteogenic differentiation in MSCs. These data demonstrate the role of serum levels in regulating osteogenesis and its importance in optimizing MSC differentiation strategies.
Highlights
-
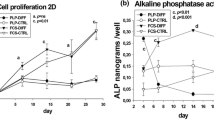
Serum levels, in addition to hypoxia, have a significant effect on MSC osteogenic differentiation.
-
Both naïve and osteogenically induced MSCs exhibit higher osteogenic markers in reduced serum.
-
MSCs deposit the most calcium under 5 % O2 in osteogenic media supplemented with 5 % FBS.
-
Standard culture conditions (21 % O2, 10 % FBS) may not be optimal for MSC osteogenic differentiation.



Similar content being viewed by others
References
Bruder, S. P., Fink, D. J., & Caplan, A. I. (1994). Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. Journal of Cellular Biochemistry, 56, 283–294.
Caplan, A. I. (2007). Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of Cellular Physiology, 213, 341–347.
Caplan, A. I. (2009). New era of cell-based orthopedic therapies. Tissue engineering Part B, Reviews, 15, 195–200.
Baksh, D., Song, L., & Tuan, R. S. (2004). Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. Journal of Cellular and Molecular Medicine, 8, 301–316.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143–147.
Park, D., Spencer, J. A., Koh, B. I., et al. (2012). Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell, 10, 259–272.
Peter, S. J., Liang, C. R., Kim, D. J., Widmer, M. S., & Mikos, A. G. (1998). Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. Journal of Cellular Biochemistry, 71, 55–62.
Jaiswal, N., Haynesworth, S. E., Caplan, A. I., & Bruder, S. P. (1997). Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Journal of Cellular Biochemistry, 64, 295–312.
Viateau, V., Guillemin, G., Bousson, V., et al. (2007). Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 25, 741–749.
Schmitt, A., van Griensven, M., Imhoff, A. B., & Buchmann, S. (2012). Application of stem cells in orthopedics. Stem Cells International, 2012, 394962.
Brighton, C. T., & Krebs, A. G. (1972). Oxygen tension of healing fractures in the rabbit. The Journal of Bone and Joint Surgery. American Volume, 54, 323–332.
R.B. Heppenstall, G. Grislis, T.K. Hunt, Tissue gas tensions and oxygen consumption in healing bone defects, Clinical orthopaedics and related research, (1975) 357–365
Ma, T., Grayson, W. L., Frohlich, M., & Vunjak-Novakovic, G. (2009). Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnology Progress, 25, 32–42.
Vater, C., Kasten, P., & Stiehler, M. (2011). Culture media for the differentiation of mesenchymal stromal cells. Acta Biomaterialia, 7, 463–477.
Dai, Y., Xu, M., Wang, Y., Pasha, Z., Li, T., & Ashraf, M. (2007). HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. Journal of Molecular and Cellular Cardiology, 42, 1036–1044.
Liu, L., Yu, Q., Lin, J., et al. (2011). Hypoxia-inducible factor-1alpha is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells and Development, 20, 1961–1971.
D’Ippolito, G., Diabira, S., Howard, G. A., Roos, B. A., & Schiller, P. C. (2006). Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone, 39, 513–522.
He, J., Genetos, D. C., Yellowley, C. E., & Leach, J. K. (2010). Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. Journal of Cellular Biochemistry, 110, 87–96.
Li, J., & Pei, M. (2012). Cell senescence: a challenge in cartilage engineering and regeneration. Tissue engineering Part B, Reviews, 18, 270–287.
Raheja, L. F., Genetos, D. C., & Yellowley, C. E. (2010). The effect of oxygen tension on the long-term osteogenic differentiation and MMP/TIMP expression of human mesenchymal stem cells. Cells, Tissues, Organs, 191, 175–184.
Hoch, A. I., Binder, B. Y., Genetos, D. C., & Leach, J. K. (2012). Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PloS One, 7, e35579.
Davis, H. E., Rao, R. R., He, J., & Leach, J. K. (2009). Biomimetic scaffolds fabricated from apatite-coated polymer microspheres. Journal of Biomedical Materials Research. Part A, 90, 1021–1031.
Kang, S. W., Lee, J. S., Park, M. S., Park, J. H., & Kim, B. S. (2008). Enhancement of in vivo bone regeneration efficacy of human mesenchymal stem cells. Journal of Microbiology and Biotechnology, 18, 975–982.
Song, L., & Tuan, R. S. (2004). Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB Journal official publication of the Federation of American Societies for Experimental Biology, 18, 980–982.
Binder, B. Y., Genetos, D. C., & Leach, J. K. (2014). Lysophosphatidic Acid protects human mesenchymal stromal cells from differentiation-dependent vulnerability to apoptosis. Tissue Engineering. Part A, 20, 1156–1164.
Potier, E., Ferreira, E., Andriamanalijaona, R., et al. (2007). Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone, 40, 1078–1087.
Caplan, A. I., & Dennis, J. E. (2006). Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry, 98, 1076–1084.
Pochampally, R. R., Smith, J. R., Ylostalo, J., & Prockop, D. J. (2004). Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood, 103, 1647–1652.
Oskowitz, A., McFerrin, H., Gutschow, M., Carter, M. L., & Pochampally, R. (2011). Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Research, 6, 215–225.
Giannoni, P., Pagano, A., Maggi, E., et al. (2005). Autologous chondrocyte implantation (ACI) for aged patients: development of the proper cell expansion conditions for possible therapeutic applications. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society, 13, 589–600.
Chacko, S. M., Ahmed, S., Selvendiran, K., Kuppusamy, M. L., Khan, M., & Kuppusamy, P. (2010). Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. American Journal of Physiology. Cell Physiology, 299, C1562–1570.
Rosova, I., Dao, M., Capoccia, B., Link, D., & Nolta, J. A. (2008). Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells, 26, 2173–2182.
Genetos, D. C., Toupadakis, C. A., Raheja, L. F., et al. (2010). Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. Journal of Cellular Biochemistry, 110, 457–467.
Volkmer, E., Kallukalam, B. C., Maertz, J., et al. (2010). Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Engineering. Part A, 16, 153–164.
Acknowledgements
This project was supported by grants from the National Institutes of Health 1R03DE021704 and the Orthopaedic Research and Education Foundation (#13-006) to JKL and by the California Institute for Regenerative Medicine UC Davis Stem Cell Training Program (CIRM T1-00006, CIRM TG2-01163) to BYB.
Disclosures
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Binder, B.Y.K., Sagun, J.E. & Leach, J.K. Reduced Serum and Hypoxic Culture Conditions Enhance the Osteogenic Potential of Human Mesenchymal Stem Cells. Stem Cell Rev and Rep 11, 387–393 (2015). https://doi.org/10.1007/s12015-014-9555-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9555-7