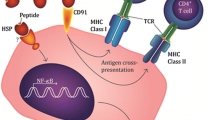
Abstract
Heat shock proteins (HSPs) are primordial and abundant molecules expressed in all cells. Publications starting in 1984 have shown that immunization of mice, rats, and frogs with purified preparations of selected HSPs isolated from cancers leads to protective immunity against the cancer used as the source of the HSP. The basis of the tumorspecific immunogenicity of these molecules lies not in the molecules themselves but in the array of peptides, including antigenic peptides chaperoned by them. These experiments and the ideas derived from them form the basis of an approach to immunotherapy for human cancer that began in 1995 and is now in full swing.
Similar content being viewed by others
References and Recommended Reading
Srivastava P: Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002, 20:395–425.
Srivastava PK, Udono H, Blachere NE, Li Z: Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 1994, 39:93–98.
Zhu X, Zhao X, Burkholder WF, et al.: Structural analysis of substrate binding by the molecular chaperone DnaK. Science 1996, 272:1606–1614.
MacAry PA, Javid B, Floto RA, et al.: HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity 2004, 20:95–106. The authors identify the minimal domain of mycobacterial hsp70 necessary for peptide binding and for antigen re-presentation by APCs. A mutation of a key amino acid in the peptide-binding domain abrogates re-presentation activity but not stimulation of chemokine and cytokine release by dendritic cells. The re-presentation activity of hsp70 is shown to be calcium dependent.
Gidalevitz T, Biswas C, Ding H, et al.: Identification of the N-terminal peptide binding site of glucose-regulated protein 94. J Biol Chem 2004, 279:16543–16552. Using molecular modeling and mutational analysis, this study identifies a peptide-binding site in the N-terminal domain of gp96 and discusses in a comparative manner some attributes of peptidebinding clefts of MHC I, hsp70, and gp96.
Baker-LePain JC, Sarzotti M, Fields TA, et al.: GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med 2002, 196:1447–1459.
Grossmann ME, Madden BJ, Gao F, et al.: Proteomics shows Hsp70 does not bind peptide sequences indiscriminately in vivo. Exp Cell Res 2004, 297:108–117.
Blond-Elguindi S, Cwirla SE, Dower WJ, et al.: Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 1993, 75:717–728.
Podack Yamazaki K, Nguyen T, Podack ER: Cutting edge: Tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol 1999, 163:5178–5182.
Massa C, Guiducci C, Arioli I, et al.: Enhanced efficacy of tumor cell vaccines transfected with secretable hsp70. Cancer Res 2004, 64:1502–1508. Recombinant hsp70 secreted from tumor cells is taken up by APCs, leading to activation of antigen-specific T cells. In addition, tumor cells engineered to secrete hsp70 induce a cytolytic T-cell response, and such tumor cells are therapeutically active against experimental lung metastases.
Tobian AA, Canaday DH, Boom WH, Harding CV: Bacterial heat shock proteins promote CD91-dependent class I MHC crosspresentation of chaperoned peptide to CD8 + T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J Immunol 2004, 172:5277–5286. This study demonstrates the ability of macrophages and dendritic cells to re-present antigenic peptides chaperoned by bacterial hsp70 family members in a CD91-dependent manner. The study also indicates that re-presentation activity of hsp70 by macrophages is largely independent of cytosolic antigen-processing machinery; in contrast, re-presentation activity of hsp70 by dendritic cells is largely dependent on cytosolic antigen-processing machinery.
Tobian AA, Canaday DH, Harding CV: Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4 + T cells. J Immunol 2004, 173:5130–5137. This study demonstrates the ability of bacterial hsp70 family members to enhance the presentation of antigenic peptides by MHC class II molecules of macrophages and dendritic cells in a CD40-independent manner.
SenGupta D, Norris PJ, Suscovich TJ, et al.: Heat shock proteinmediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J Immunol 2004, 173:1987–1993. This paper demonstrates the ability of gp96 complexed to an extended HIV peptide to stimulate T-cell clones specific for multiple epitopes contained within the peptide.
Kumaraguru U, Gierynska M, Norman S, et al.: Immunization with chaperone-peptide complex induces low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J Virol 2002, 76:136–141.
Kumaraguru U, Suvas S, Biswas PS, et al.: Concomitant helper response rescues otherwise low avidity CD8 + memory CTLs to become efficient effectors in vivo. J Immunol 2004, 172:3719–3124. This study demonstrates that memory CD8 responses induced by hsp70 complexed to a herpes virus MHC class I epitope can be enhanced by co-immunization with HSP70 complexed to a class II viral epitope.
Doody AD, Kovalchin JT, Mihalyo MA, et al.: Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8(+) T cell effector function. J Immunol 2004, 172:6087–6092. This paper confirms the ability of gp96 to prime CD8 T-cell response to a viral antigen, and, more importantly, demonstrates priming of antigen-specific CD4 response. The qualitative nature of the two types of responses is demonstrated to differ. The CD8+ T cells elicited by immunization with gp96-peptide complexes are mature effectors, whereas the CD4+ T cells are not.
Rapp UK, Kaufmann SH: DNA vaccination with gp96-peptide fusion proteins induces protection against an intracellular bacterial pathogen. Int Immunol 2004, 16:597–605. The authors demonstrate the ability of DNA-based mammalian HSP-antigen fusion constructs to induce T-cell response and protective immunity. gp96 fused to bacterial antigens is shown to induce effector CD8 T cells, which confer resistance to challenge with lethal doses of bacteria.
Wang XY, Kazim L, Repasky EA, Subjeck JR: Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer 2003, 105:226–231. This study shows the therapeutic benefit of tumor-derived preparations of the HSP grp170 in a mouse model of metastatic melanoma.
Manjili MH, Wang XY, Chen X, et al.: HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol 2003, 171:4054–4061. In a mouse model of spontaneous HER-2/neu-positive breast cancer, immunization with non-covalent complexes of hsp110 and the intracellular domain of HER-2/neu elicits antigen-specific T-cell and antibody responses and delays tumor progression. 20. Ito A, Shinkai M, Honda H, et al.: Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol Immunother 2003, 52:80-88. This study indicates that in vivo hypothermia localized to tumor tissue results in tumor necrosis, release of immunogenic hsp70, and protective tumor immunity.
Graner MW, Zeng Y, Feng H, Katsanis E: Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother 2003, 52:226–234. In this study, tumor lysates subjected to a process that enriches for at least four HSPs are demonstrated to induce tumor regression and prolong survival in a variety of mouse tumor therapy models. Adoptively transferred dendritic cells that had been pulsed in vitro with the chaperone-rich cell lysates were shown to be particularly effective in treatment of preexisting disease.
Binder RJ, Han DK, Srivastava PK: CD91: a receptor for heat shock protein gp96. Nat Immunol 2000, 1:151–155.
Basu S, Binder RJ, Ramalingam T, Srivastava PK: CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001, 14:303–313.
Binder RJ, Vatner R, Srivastava P: The heat-shock protein receptors: some answers and more questions. Tissue Antigens 2004, 64:442–445. This review provides a complete listing and critical analysis of all attributed HSP receptors to date.
Binder RJ, Srivastava PK: Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci U S A 2004, 101:6128–6133. Using genetic tools, this study demonstrates that the loss of CD91 expression by APCs leads to corresponding loss of the ability of those cells to bind to gp96 and to re-present gp96-chaperoned peptides.
Orr AW, Pedraza CE, Pallero MA, et al.: Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol 2003, 161:1179–1189. This study provides additional evidence for direct interaction between calreticulin and CD91.
Stebbing J, Gazzard B, Kim L, et al.: The heat-shock protein receptor CD91 is up-regulated in monocytes of HIV-1-infected ‘true’ long-term nonprogressors. Blood 2003, 101:4000–4004. This study shows a positive correlation between expression of the HSP receptor CD91 and the maintenance of long-term nonprogression in HIV-infected individuals.
Stebbing J, Gazzard B, Portsmouth S, et al.: Disease associated dendritic cells respond to disease-specific antigens through the common heat shock protein receptor. Blood 2003, 102:1806–1814. These experiments indicate that HSP-peptide complexes present in Kaposi sarcoma lysates play an important role in stimulating T-cell immunity to Kaposi antigens. The study also provides support for the role of CD91 on dendritic cells in antigen presentation to T lymphocytes.
Berwin B, Hart JP, Rice S, et al.: Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigenpresenting cells. EMBO J 2003, 22:6127–6136. This study, along with Berwin et al. [30], suggests a role for scavenger receptors SR-A and SREC-I on mouse macrophage in re-presentation of peptides chaperoned by gp96 and calreticulin. The case is made on the basis of competition of HSP uptake by fucoidin and acetylated low-density lipoprotein, by the use of SR-A knockout mice, and by transfection of the gene encoding the putative receptor into CHO cells.
Berwin B, Delneste Y, Lovingood RV, et al.: SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem 2004, 279:51250–51257.
Radsak MP, Hilf N, Singh-Jasuja H, et al.: The heat shock protein gp96 binds to human neutrophils and monocytes and stimulates effector functions. Blood 2003, 101:2810–2815. This article identifies a new role for the HSP gp96 in binding to and activating specialized cells that play an important role in wound healing. The study indicates that gp96 naturally released from damaged tissue can recruit cells to the damaged site to initiate the wound healing process.
Castelli C, Ciupitu AM, Rini F, et al.: Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res 2001, 61:222–227.
Noessner E, Gastpar R, Milani V, et al.: Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol 2002, 169:5424–5432.
Staib F, Distler M, Bethke K, et al.: Cross-presentation of human melanoma peptide antigen MART-1 to CTLs from in vitro reconstituted gp96/MART-1 complexes. Cancer Immun 2004, 4:3.
Belli F, Testori A, Rivoltini L, et al.: Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol 2002, 20:4169–4180.
Mazzaferro V, Coppa J, Carrabba MG, et al.: Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res 2003, 9:3235–3245. In this study more than half of the 29 patients who received HSPPC-96 demonstrated significant immunologic response, which not only appeared to be correlated with clinical response but also was found to be an independent factor for prognosis.
Rivoltini L, Castelli C, Carrabba M, et al.: Human tumorderived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinomaspecific T cells. J Immunol 2003, 171:3467–3474. This study further demonstrates the ability of autologous, patient-derived gp96-peptide complexes to elicit tumor-specific T-cell response in HSP-vaccinated cancer patients.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Srivastava, P.K. Immunotherapy for human cancer using heat shock protein-peptide complexes. Curr Oncol Rep 7, 104–108 (2005). https://doi.org/10.1007/s11912-005-0035-8
Issue Date:
DOI: https://doi.org/10.1007/s11912-005-0035-8