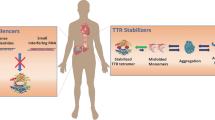
Abstract
Transthyretin amyloidosis (ATTR) is either a hereditary disease related to a mutation in the transthyretin gene that leads to neuropathy and/or cardiomyopathy or an acquired disease of the elderly that leads to restrictive cardiomyopathy. The prevalence of this disease is higher than once thought and awareness is likely to increase amongst physicians and in particular cardiologists. Until recently there have been no treatment options for this disease except to treat the heart failure with diuretics and the neuropathy symptomatically. However, there are several emerging pharmacologic therapies designed to slow or stop the progression of ATTR. This article reviews novel therapeutic drugs that work at different points in the pathogenesis of this disease attempting to change its natural history and improve outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–96.
Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: a comprehensive review. Arch Intern Med. 2006;166(17):1805–13.
Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347–61.
Esplin BL, Gertz MA. Current trends in diagnosis and management of cardiac amyloidosis. Curr Probl Cardiol. 2013;38(2):53–96. This is an outstanding and comprehensive review of cardiac amyloidsois with guest commentary by Rodney Falk.
Dubrey SW et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141–57.
Dungu JN et al. Cardiac transthyretin amyloidosis. Heart. 2012;98(21):1546–54. Excellent review of Transhyretin Cardiac amyloidosis with multiple images and instructive figures.
Zeldenrust SR, Cooper LT. Getting to the heart of the matter: cardiac involvement in transthyretin-related amyloidosis. Eur Heart J. 2013;34(7):483–5.
Rapezzi C et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120(13):1203–12.
Nomenclature. Nomenclature Committee of IUB. IUB–IUPAC Joint Commission on Biochemical Nomenclature. Arch Biochem Biophys. 1981;206(2):458–62.
Robbins J. Transthyretin from discovery to now. Clin Chem Lab Med. 2002;40(12):1183–90.
Arruda-Olson AM et al. Genotype, echocardiography, and survival in familial transthyretin amyloidosis. Amyloid. 2013;20(4):263–8.
Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–300. Comprehensive up-to date review of transthyretin cardiac amyloidosis outlining epidemiology, pathogenesis, diagnosis, and treatment. It includes a summary of emerging treatments.
Ihse E et al. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol. 2008;216(2):253–61.
Lobato L. Portuguese-type amyloidosis (transthyretin amyloidosis, ATTR V30M). J Nephrol. 2003;16(3):438–42.
Jacobson DR et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336(7):466–73.
Yamashita T et al. A prospective evaluation of the transthyretin Ile122 allele frequency in an African-American population. Amyloid. 2005;12(2):127–30.
Buxbaum J et al. Transthyretin V122I in African Americans with congestive heart failure. J Am Coll Cardiol. 2006;47(8):1724–5.
Ruberg FL et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;164(2):222–8. e1.
Pitkanen P, Westermark P, Cornwell 3rd GG. Senile systemic amyloidosis. Am J Pathol. 1984;117(3):391–9.
Pinney JH et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2(2):e000098.
Ng B et al. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165(12):1425–9.
Cornwell 3rd GG et al. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983;75(4):618–23.
Tanskanen M et al. Senile systemic amyloidosis affects 25 % of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40(3):232–9.
Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14(30):3219–30.
Bulawa CE et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109(24):9629–34.
Gillmore JD et al. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br J Haematol. 2010;148(5):760–7.
Benson MD. Pathogenesis of transthyretin amyloidosis. Amyloid. 2012;19 Suppl 1:14–5. A concise two page summary of the pathogenesis of transhyretin amyloid, summarizing what we know and what we don't know. Excellent read.
Zhao L, Buxbaum JN, Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry. 2013;52(11):1913–26.
Holmgren G et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet. 1991;40(3):242–6.
Pomfret EA et al. Effect of orthotopic liver transplantation on the progression of familial amyloidotic polyneuropathy. Transplantation. 1998;65(7):918–25.
Sharma P et al. Outcome of liver transplantation for familial amyloidotic polyneuropathy. Liver Transpl. 2003;9(12):1273–80.
Ericzon BG et al. Liver transplantation halts the progress of familial amyloidotic polyneuropathy. Transplant Proc. 1995;27(1):1233.
Suhr OB et al. Liver transplantation in familial amyloidotic polyneuropathy. Follow-up of the first 20 Swedish patients. Transplantation. 1995;60(9):933–8.
Yamamoto S et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant. 2007;7(11):2597–604.
Okamoto S et al. Prognostic value of pre-transplant cardiomyopathy in Swedish liver transplanted patients for familial amyloidotic polyneuropathy. Amyloid. 2011;18 Suppl 1:171–3.
Nelson LM et al. Long-term outcome in patients treated with combined heart and liver transplantation for familial amyloidotic cardiomyopathy. Clin Transplant. 2013;27(2):203–9.
Hamour IM et al. Heart transplantation for homozygous familial transthyretin (TTR) V122I cardiac amyloidosis. Am J Transplant. 2008;8(5):1056–9.
Lachmann HJ. A new era in the treatment of amyloidosis? N Engl J Med. 2013;369(9):866–8. A very insightful editorial in response to the New England Journal publication about small interfering RNA treatment in TTR amyloid. This summarizes the current issues and strategies very well.
Coelho T et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–29. Along with antisense oligonucleotides, this is a major breakthrough and publication in the field. Getting published in the New England Journal of Medicine has brought transthyretin amyloidosis into greater awareness amongst physicians.
Malik R, Roy I. Making sense of therapeutics using antisense technology. Expert Opin Drug Discov. 2011;6(5):507–26.
Ackermann EJ et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19 Suppl 1:43–4.
Adamski-Werner SL et al. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47(2):355–74.
Gales L et al. Human transthyretin in complex with iododiflunisal: structural features associated with a potent amyloid inhibitor. Biochem J. 2005;388(Pt 2):615–21.
Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13(4):236–49.
Castano A et al. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012;18(6):315–9.
Berk JL et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–67. This is the only randomized placebo-controlled trial in transthyretin amyloidosis that has met its primary endpoint. This landmark clincial trial will likely lead to the approval of Diflunisal for the indication of FAP.
Coelho T et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–92. This was the first randomized placebo-controlled trial in transthyretin amyloidosis. Although the primary endpoint was not met, the results led to the approval of Tafamdis in Europe.
Cardoso I, Saraiva MJ. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J. 2006;20(2):234–9.
Ward JE et al. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118(25):6610–7.
Cardoso I et al. Synergy of combined doxycycline/TUDCA treatment in lowering Transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med. 2010;8:74.
Obici L et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid. 2012;19 Suppl 1:34–6.
Ferreira N et al. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009;583(22):3569–76.
Ferreira N, Saraiva MJ, Almeida MR. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011;585(15):2424–30.
Palhano FL et al. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J Am Chem Soc. 2013;135(20):7503–10.
Miyata M et al. The crystal structure of the green tea polyphenol (-)-epigallocatechin gallate-transthyretin complex reveals a novel binding site distinct from the thyroxine binding site. Biochemistry. 2010;49(29):6104–14.
Mereles D et al. Effects of the main green tea polyphenol epigallocatechin-3-gallate on cardiac involvement in patients with AL amyloidosis. Clin Res Cardiol. 2010;99(8):483–90.
Kristen AV et al. Green tea halts progression of cardiac transthyretin amyloidosis: an observational report. Clin Res Cardiol. 2012;101(10):805–13.
Pepys MB et al. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979;38(2):284–93.
Hohenester E et al. Crystal structure of a decameric complex of human serum amyloid P component with bound dAMP. J Mol Biol. 1997;269(4):570–8.
Botto M et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3(8):855–9.
Bodin K et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468(7320):93–7.
Compliance with Ethics Guidelines
Conflict of Interest
Mazen Hanna received a one-time consultant fee from Pfizer in April 2012 regarding input for the drug Tafamadis. He is also the local principal investigator of the Tafamadis Cardiomyopathy Trial and is a member of the Scientific Board for THAOS (Transthyretin Outcomes Survey), both sponsored by Pfizer.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanna, M. Novel Drugs Targeting Transthyretin Amyloidosis. Curr Heart Fail Rep 11, 50–57 (2014). https://doi.org/10.1007/s11897-013-0182-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-013-0182-4