Abstract
Objective
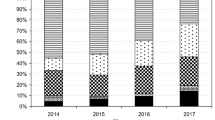
Use of meropenem in our hospital has doubled in recent years. An audit in 2013 showed that although initiation of therapy with meropenem was generally appropriate, therapy was rarely subsequently reviewed and de-escalated where appropriate. Therefore, a structured stewardship initiative focussed on meropenem de-escalation was developed.
Methods
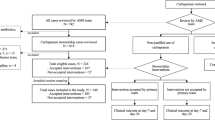
A local guideline for review and de-escalation of meropenem was developed and approved by the Antimicrobial Stewardship Team. The guideline outlined clinical and microbiological criteria which when met should lead to recommendation for meropenem de-escalation. Implementation of the guideline was piloted for a period of 4 weeks by a consultant microbiologist and an antimicrobial pharmacist. Days of meropenem use and crude mortality in those in whom de-escalation was implemented were compared with those where de-escalation was not recommended or was recommended but not implemented.
Results
Thirty-three patients were reviewed. Overall, a recommendation to de-escalate from meropenem to a specified alternative antibiotic was made for 18 (55 %) patients. This advice was followed for 12 (36 %) patients. The median days of meropenem use in patients where meropenem was de-escalated was 4.5 days (range 2–19) compared with 14 days (range 6–84) where de-escalation was not recommended or the recommendation was not implemented. There was no statistically significant difference in crude mortality between patients de-escalated from meropenem and those where meropenem was continued.
Conclusion
This pilot study suggests that targeted carbapenem de-escalation stewardship activity based on pre-determined criteria, while labour intensive, can effectively and safely reduce meropenem use in the acute hospital setting.
Similar content being viewed by others
References
World Health Organization (2015) Global action plan on antimicrobial resistance (2015). ISBN 978 92 4 1509763 WHO http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf?ua=1. Accessed 18 Feb 2016
European Centre for Disease Prevention and Control (2014) Surveillance of antimicrobial consumption in Europe 2012. ECDC, Stockholm
Petty NK, Ben Zakoura NL, Stanton-Cooka M et al (2014) Global dissemination of a multidrug resistant Escherichia coli clone. PNAS 111:5694–5699
Munoz-Price LS, Poirel L, Bonomo RA et al (2013) Clinical epidemiology of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 9:785–796. doi:10.1016/S1473-3099(13)70190-7
Vogelaers D, De Bels D, Forêt F et al (2010) ANTHICUS study investigators. Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates-a multicentre, observational survey in critically ill patients. Int J Antimicrob Agents 35(4):375–381. doi:10.1016/j.ijantimicag.2009.11.015 (Epub 2010 Feb 1)
Oza A, Burns K, Cunney R (2014) Carbapenem use in hospitals has doubled in the last five years. Epi-Insight, disease surveillance report of HPSC, Ireland, November. 15 (11)
Health Protection Surveillance Centre Ireland, Irish EARS-Net Steering Group (2015) EARS-net report, quarters 1–4 2014. March 2015. http://www.hpsc.ie/A-Z/MicrobiologyAntimicrobialResistance/EuropeanAntimicrobialResistanceSurveillanceSystemEARSS/EARSSSurveillanceReports/2014Reports/Z/MicrobiologyAntimicrobialResistance/EuropeanAntimicrobialResistanceSurveillanceSystemEARSS/EARSSSurveillanceReports/2014Reports/
Morris D, O’Connor M, Izdebski R et al (2016) Dissemination of clonally related multidrug-resistant Klebsiella pneumonia in Ireland. Epidemiol Infect 144(2):443–448
Cai Y, Shek PY, Teo I et al (2016) A multidisciplinary antimicrobial stewardship programme safely decreases the duration of broad-spectrum antibiotic prescription in Singaporean adult renal patients. Int J Antimicrob Agents 47(1):91–96. doi:10.1016/j.ijantimicag.2015.10.021 (Epub 2015 Nov 26)
Acknowledgments
We thank all members of the multidisciplinary antimicrobial stewardship team for contribution to and approval of the meropenem de-escalation guideline and the clinical ward pharmacists for identifying and referring patients on meropenem to the review team.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work did not involve research involving human participants and/or animals or require informed consent.
Funding
This stewardship initiative was developed and pilot implemented as part of ongoing stewardship programme activities without additional funding.
Transparency declarations
The authors have no conflicts of interest that are relevant to the content of this study.
Rights and permissions
About this article
Cite this article
Ni Riain, U., Tierney, M., Doyle, C. et al. Targeted de-escalation rounds may effectively and safely reduce meropenem use. Ir J Med Sci 186, 729–732 (2017). https://doi.org/10.1007/s11845-016-1504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-016-1504-9