Abstract
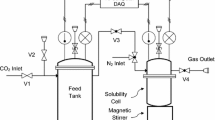
The CO2 absorption rate into aqueous N-methyldiethanolamine solutions was measured using a stirred cell with a flat gas-liquid interface. The measurements were performed in the temperature range of 293.15 to 333.15 K for various amine concentrations and CO2 partial pressures. A numerical model of mass-transfer with complex chemical reactions based on the film theory was developed to interpret the experimental results. The model predictions have been found to be in good agreement with the experimental values of CO2 absorption rates. A comparison is made between the enhancement factor predicted from the detailed model and the approximate solution of mass transfer equations with chemical reaction. The numerical results indicate that under the present experimental conditions, the effect of the reaction between CO2 and OH− on the observed mass transfer rates is negligible. The detailed mass transfer model was used for simulating the CO2 absorption process in terms of the enhancement factor under a variety of operating conditions.
Similar content being viewed by others
References
A. L. Kohl and R. B. Nielsen, Gas Purification (5th Ed.), Gulf Publishing Co., Houston (1997).
R. J. Notz, I. Tönnies, N. McCann, G. Scheffknecht and H. Hasse, Chem. Eng. Technol., 34(2), 163 (2011).
R. J. Notz, N. Asprion, I. Clause and H. Hasse, Chem. Eng. Res. Des., 85(4), 510 (2007).
N. Haimour, A. Bidarian and O. Sandall, Chem. Eng. Sci., 42, 1393 (1987).
R. J. Littel, W. P.M. Van Swaaij and G. F. Versteeg, AIChE J., 36(11), 1633 (1990).
R. A. Tomsej and F. D. Otto, Chem. Eng. Sci., AIChE J., 35(5), 573 (1989).
F. Pani, A. Gaunand, R. Cadours, C. Bouallou and D. Richon, J. Chem. Eng. Data, 42(2), 353 (1997).
J.-J. Ko and M.-H. Li, Chem. Eng. Sci., 55(9), 4139 (2000).
W. Moniuk and R. Pohorecki, In ynieria Chemiczna i Procesowa, 21(1), 183 (2000).
Jamal, A. Meisen, C. Jim Lim, Chem. Eng. Sci., 61, 6571 (2006).
Jamal, A. Meisen, C. Jim Lim, Chem. Eng. Sci., 61, 6590 (2006).
A. Benamor and M. K. Aroua, J. Chem. Eng., 24, 16 (2007).
P. D. Vaidya and E.Y. Kenig, Chem. Eng. Technol., 30(11), 1467 (2007).
T. L. Donaldson and Y. N. Nguyen, Ind. Eng. Chem. Fundam., 19, 260 (1980).
D. A. Glasscock and G. T. Rochelle, AIChE J., 35, 1271 (1989).
E.B. Rinker, S. S. Ashour and O. C. Sandall, Chem. Eng. Sci., 50, 755 (1995).
R. Cadours and C. Bouallou, Ind. Eng. Chem. Res., 37(3), 1063 (1998).
A. Kabouche, A. H. Meniai and M. Bencheikk-lehocine, Chem. Eng. Technol., 28(1), 67 (2005).
D.W. Marguardt, J. Soc. Indust. Appl. Math., 11, 431 (1963).
R. Zarzycki and A. Chacuk, Absorption: Fundamentals and applications, Pergamon Press, Oxford (1993).
H. Kierzkowska-Pawlak and A. Chacuk, Chem. Eng. J., 168(1), 367 (2011).
G. F. Versteeg and W. P.M. Van Swaaij, J. Chem. Eng. Data, 33(1), 29 (1988).
H.A. Al-Ghawas, D. P. Hagewiesche, G. Ruiz-Ibanez and O. C. Sandall, J. Chem. Eng. Data, 34(4), 385 (1989).
R. Cadours, H. Bouallou and A. Gaunand, Ind. Eng. Chem. Res., 36, 5384 (1997).
R.W. Pinsent, L. Pearson and F. J.W. Roughton, Trans. Faraday Soc., 52, 1512 (1956).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kierzkowska-Pawlak, H., Chacuk, A. Numerical simulation of CO2 absorption into aqueous methyldiethanolamine solutions. Korean J. Chem. Eng. 29, 707–715 (2012). https://doi.org/10.1007/s11814-011-0244-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-011-0244-9