Abstract
Background
Bariatric surgery improves obesity and ameliorates glucose tolerance. This study was conducted to evaluate circadian clocks, gluconeogenesis, and glucose transport changes in hepatic and intestinal tissues after duodenal–jejunal bypass (DJB) surgery in a rat model.
Methods
Twenty-five rats were randomly assigned to either sham group (10 rats) or DJB group (15 rats). Food intake, body weight, blood glucose, and serum insulin levels were measured. Quantitative RT-PCR, immunoblot, and immunohistochemistry were used to analyze genes and proteins in the liver and intestine.
Results
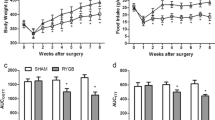
Food intake and body weight were not different between sham and DJB groups. Blood glucose level was significantly lower in the DJB group compared with that in the sham group. Although not significant, serum insulin level showed an increased tendency in DJB group. DJB induced marked expressions of glucose transporter-2 (GLUT2) in the liver and GLUT2 and sodium-dependent glucose transporter-1 (SGLT1) in the intestine. Gluconeogenic enzymes [phosphoenolpyruvate carboxykinase-1 (Pck1) and glucose-6-phosphatase (G6Pase)] decreased in the liver and increased in the intestine of the DJB group. Circadian transcription factor cryptochrome-1 (Cry1) increased in the liver and decreased in the intestine of the DJB group. Another circadian transcription factor period-2 (Per2) also increased in the liver and decreased in the intestine of the DJB group.
Conclusion
In conclusion, this study suggests the possibility that Cry1 and Per2 may mediate decreased gluconeogenesis in the liver and increased gluconeogenesis in the intestine of the DJB group.



Similar content being viewed by others
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9.
Cummings DE, Overduin J, Foster-Schubert KE, et al. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis. 2007;3:109–15.
Moo TA, Rubino F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:153–8.
Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25.
Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–11.
Mithieux G. A novel function of intestinal gluconeogenesis: central signaling in glucose and energy homeostasis. Nutrition. 2009;25:881–4.
Quercia I, Dutia R, Kotler DP. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40:87–94.
Li B, Lu Y, Srikant CB, et al. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal–jejunal bypass surgery in Zucker fatty rats. Am J Physiol Gastrointest Liver Physio. 2013;304:G635–45.
Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–10.
Zheng Y, Scow JS, Duenes JA, et al. Mechanisms of glucose uptake in intestinal cell lines: Role of GLUT2. Surgery. 2012;151:13–25.
Hoogerwerf WA, Hellmich HL, Cornelissen G, et al. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenteology. 2007;133:1250–60.
Hatori M, Panda S. CRY links the circadian clock and CREB-mediated gluconeogenesis. Cell Res. 2010;20:1285–8.
Zhang EE, Liu Y, Dentin R, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–6.
Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11.
Mingrone G, Panunzi S, Gaetano AD, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Moriya R, Shirakura T, Ito J, et al. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358–65.
Jurowich CF, Rikkala PR, Thalheimer A, et al. Duodenal–jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann Surg. 2013;258:89–97.
Yan S, Sun F, Li Z, et al. Reduction of intestinal electrogenic glucose absorption after duodenojejunal bypass in a mouse mode. Obes Surg. 2013;23:1361–9.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal–jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18:950–5.
Acknowledgments
This work was supported by the research fund of Hanyang University (HY-20010-MC).
Conflict of Interest
Mikyung Kim, Young Gil Son, Yu Na Kang, Tae Kyung Ha, and Eunyoung Ha have no conflicts of interest or other financial ties to disclose.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. Kim and Y.G. Son contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, M., Son, Y.G., Kang, Y.N. et al. Changes in Glucose Transporters, Gluconeogenesis, and Circadian Clock after Duodenal–Jejunal Bypass Surgery. OBES SURG 25, 635–641 (2015). https://doi.org/10.1007/s11695-014-1434-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1434-4