Abstract
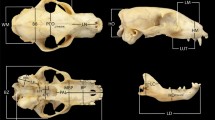
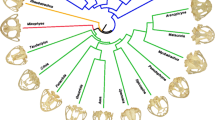
New World leaf-nosed bats (Family Phyllostomidae) display incredible craniofacial diversity that is associated with their broad range of dietary preferences. The short and broad palates of highly frugivorous bats are functionally linked to high bite forces, and the long and narrow palates of nectarivorous bats to flower feeding. Although the functional correlates and evolutionary history of shape variation in phyllostomid palates are beginning to be understood, the specific developmental processes that govern palate diversification remain unknown. To begin to resolve this issue, this study quantified palate morphology in seven phyllostomid species from a range of developmental stages and in adults. This sample includes species with short and broad, long and narrow, and intermediate palate shapes, and thereby covers the range of palate shapes displayed by phyllostomids. Results indicate that while initial palate shape (i.e., width vs. length) varies among species, the pattern of this variation does not match that observed in adults. In contrast, the relative growth of palate width and length in developing phyllostomids and the ratio of these axes in adults are significantly correlated. These and other results suggest that evolutionary alterations in patterns of palate growth have governed the diversification of palate shapes in adult phyllostomids. This implies that the diverse palate shapes of phyllostomids are the result of relatively subtle evolutionary changes in later rather than earlier development events.


Similar content being viewed by others
References
Abzhanov, A., Kuo, W. P., Hartman, C. G., Grant, B. R., Grant, P. R., & Tabin, C. J. (2006). The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature, 442(7102), 563–567.
Abzhanov, A., Protas, M., Grant, B. R., Grant, P. R., & Tabin, C. J. (2004). Bmp4 and morphological variation of beaks in Darwin’s finches. Science, 305, 1462–1465.
Albertson, R. C., Streelman, J. T., Kocher, T. D., & Yelick, P. C. (2005). Integration and evolution of the cichlid mandible: The molecular basis of alternate feeding strategies. Proceedings of the National Academy of Sciences of the United States of America 102(45):16287–92.
Albertson, R. C., Yan, Y. L., Titus, T. A., Pisano, E., Vacchi, M., Yelick, P. C., et al. (2010). Molecular pedomorphism underlies craniofacial skeletal evolution in Antarctic notothenioid fishes. BMC Evolutionary Biology, 10, 4.
Bininda-Emonds, O. R. P., Jeffery, J. E., & Richardson, M. K. (2003). Inverting the hourglass: Quantitative evidence against the phylotypic stage in vertebrate development. Proceedings of the Royal Society B, 270, 341–346.
Cheverud, J. M. (1982). Relationships among ontogenetic, static, and evolutionary allometry. American Journal of Physical Anthropology, 59(2), 139–149.
Cretekos, C. J., Weatherbee, S. D., Chen, C. H., Badwaik, N. K., Niswander, L., Behringer, R. R., et al. (2005). Embryonic staging system for the short-tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive-bred animals. Developmental Dynamics, 233, 721–738.
Damuth, J., & MacFadden, B. J. (1990). Body Size in mammalian paleobiology (p. 409). New York: Cambridge University Press.
Domazet-Loso, T., & Tautz, D. (2010). A phylogenetically based transcriptome age index mirrors ontogenetic divergence patterns. Nature, 468(7325), 815–818.
Drake, A. G., & Klingenberg, C. P. (2008). The pace of morphological change: Historical transformation of skull shape in St Bernard dogs. Proceedings of Biological Sciences, 275(1630), 71–76.
Dumont, E. R. (1997). Cranial shape in fruit, nectar and exudate feeders. American Journal of Physical Anthropology, 102, 187–202.
Dumont, E. R. (1999). The effect of food hardness on feeding behaviour in frugivorous bats (Phyllostomidae): An experimental study. Journal of Zoology, 248, 219–229.
Dumont, E. R. (2004). Patterns of diversity in cranial shape among plant-visiting bats. Acta Chiropterol, 6(1), 59–74.
Dumont, E. R. (2006). The correlated evolution of cranial morphology and feeding behavior in new world fruit bats. In A. Zubaid, G. F. McCracken, & T. H. Kunz (Eds.), Functional and evolutionary ecology of bats (pp. 160–177). Chicago: University of Chicago Press.
Dumont, E. R. (2007). Feeding mechanisms in bats: Variation within the constraints of flight. Integrative and Comparative Biology, 47(1), 137–146.
Dumont, E. R., Davalos, L. M., Goldberg, A., Santana, S. E., Rex, K., & Voigt, C. C. (2012). Morphological innovation, diversification and invasion of a new adaptive zone. Proceedings of Biological Sciences, 279(1734), 1797–1805.
Ferrarezzi, H., & Gimenez, E. D. A. (1996). Systematic patterns and the evolution of feeding habits in Chiroptera (Archonta: Mammalia). Journal of Computational Biology, 1, 75–94.
Freeman, P. W. (1988). Frugivorous and animalivorous bats (Microchiroptera): Dental and cranial adaptations. Biological Journal of the Linnean Society, 33, 249–272.
Freeman, P. W. (1998). Form, function, and evolution in skulls and teeth of bats. In T. H. Kunz & P. A. Racey (Eds.), Bat biology and conservation (pp. 140–156). Washington, DC: Smithsonian Institution Press.
Galis, F., & Metz, J. A. J. (2001). Testing the vulnerability of the phylotypic stage: On modularity and evolutionary conservation. Journal of Experimental Zoology Part B, 291, 195–204.
Galis, F., van Alphen, J. J. M., & Metz, J. A. J. (2001). Why five fingers? Evolutionary constraints on digit numbers. Trends in Ecology & Evolution, 16(11), 637–646.
Gardner, A. L. (1977). Feeding habits. In R. J. Baker, J. K. Jones, & D. C. Carter (Eds.), Biology of bats of the new world family Phyllostomidae, part 2 (pp. 293–350). Lubbock: Texas Tech Press.
Garfield, D. A., & Wray, G. A. (2009). Comparative embryology without a microscope: Using genomic approaches to understand the evolution of development. Journal of Biology, 8, 65.
Goswami, A. (2006). Morphological integration in the carnivoran skull. Evolution, 60(1), 169–183.
Grant, P. R., & Grant, B. R. (2006). Evolution of character displacement in Darwin’s finches. Science, 313(5784), 224–226.
Harris, M. P. (2012). Comparative genetics of postembryonic development as a means to understand evolutionary change. Journal of Applied Ichthyology, 28(3), 306–315.
Howell, D. J., & Hodgkin, N. (1976). Feeding adaptations in the hairs and tongues of nectar-feeding bats. Journal of Morphology, 148, 329–336.
Kalinka, A. T., Varga, K. M., Gerrard, D. T., Preibisch, S., Corcoran, D. L., Jarrells, J., et al. (2010). Gene expression divergence recapitulates the developmental hourglass model. Nature, 468(7325), 811–814.
Klingenberg, C. K. (1998). Heterochrony and allometry: The analysis of evolutionary change in ontogeny. Biological Reviews, 73, 79–123.
Klingenberg, C. P. (2008). Morphological integration and developmental modularity. Annual Review of Ecology Evolution and Systematics, 39, 115–132.
Klingenberg, C. P. (2010). There’s something afoot in the evolution of ontogenies. BMC Evolutionary Biology, 10, 221.
Mallarino, R., Grant, P. R., Grant, B. R., Herrel, A., Kuo, W. P., & Abzhanov, A. (2011). Two developmental modules establish 3D beak-shape variation in Darwin’s finches. Proceedings of the National Academy of Sciences of the United States of America, 108(10), 4057–4062.
Myers, P., Espinoza, R., Parr, S., Jones, T., Hammond, G. S., & Dewey, T. A. (2013). The animal diversity web (online). Accessed at http://animaldiversity.org.
Parsons, K. J., Cooper, W. J., & Albertson, R. C. (2011). Modularity of the oral jaws is linked to repeated changes in the craniofacial shape of African cichlids. International Journal of Evolutionary Biology, 2011, 641501.
Raff, R. A. (1996). The shape of life: Genes, development, and the evolution of animal form. Chicago: University of Chicago Press.
Richardson, M. K. (1999). Vertebrate evolution: The developmental origins of adult variation. BioEssays, 21, 604–613.
Roberts, R. B., Hu, Y., Albertson, R. C., & Kocher, T. D. (2011). Craniofacial divergence and ongoing adaptation via the hedgehog pathway. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13194–13199.
Roux, J., & Robinson-Rechavi, M. (2008). Developmental constraints on vertebrate genome evolution. PLoS Genetics, 4(12), 1–10.
Sanger, T. J., Mahler, D. L., Abzhanov, A., & Losos, J. B. (2012). Roles for modularity and constraint in the evolution of cranial diversity among anolis lizards. Evolution, 66(5), 1525–1542.
Santana, S. E., Dumont, E. R., & Davis, J. L. (2010). Mechanics of bite force production and its relationship to diet in bats. Functional Ecology, 24(4), 776–784.
Santana, S. E., Grosse, I. R., & Dumont, E. R. (2012). Dietary hardness, loading behavior, and the evolution of skull form in bats. Evolution, 66(8), 2587–2598.
Schluter, D., Price, T. D., & Grant, P. R. (1985). Ecological character displacement in Darwin’s finches. Science, 227(4690), 1056–1059.
Sears, K. E., Finarelli, J. A., Flynn, J. J., & Wyss, A. (2008). Morphometric estimators of body mass in new world monkeys (Platyrrhini, Anthropoidea, Primates), with consideration of the miocene-aged Chilecebus carrascoensis. American Museum Novitiates, 3617, 1–29.
Sears, K. E., Goswami, A., Flynn, J. J., & Niswander, L. A. (2007). The correlated evolution of Runx2 tandem repeats, transcriptional activity, and facial length in carnivora. Evolution and Development, 9(6), 555–565.
Simmons, N. B. (2005). Order Chiroptera. In D. E. Wilson & D. M. Reeder (Eds.), Mammal species of the world: A taxonomic and geographic reference (pp. 313–529). Baltimore: Johns Hopkins University Press.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry (p. 880). New York: W.H. Freeman and Company.
Sorensen, D. W., Butkus, C., & Cooper, L. N. (in review). The role of development in palate variation in leaf-nosed bats (Order Chiroptera, Family Phyllostomidae).
Weston, E. M. (2003). Evolution of ontogeny in the hippopotamus skull: Using allometry to dissect developmental change. Biological Journal of the Linnean Society, 80, 625–638.
Wilmore, K. E., Leamy, L. J., & Hallgrimsson, B. (2006). Effects of developmental and functional interactions on mouse cranial variability through late ontogeny. Evol Dev, 8(6), 550–567.
Wu, P., Jiang, T. X., Shen, J. Y., Widelitz, R. B., & Chuong, C. M. (2006). Morphoregulation of avian beaks: Comparative mapping of growth zone activities and morphological evolution. Developmental Dynamics, 235(5), 1400–1412.
Wu, P., Jiang, T. X., Suksaweang, S., Widelitz, R. B., & Chuong, C. M. (2004). Molecular shaping of the beak. Science, 305(5689), 1465–1466.
Acknowledgments
I would like to thank B. Stanley and the rest of the staff of the mammals collection at the Field Museum of Natural History for providing access to the cleared and stained bat specimens that are the foundation of this study, and to Drs. J. Marcot, B. Dumont, and S. Swartz for discussion of ideas. NIH NRSA F32 HD050042-01 and NSF IOS-1257873 supported this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sears, K.E. Differences in Growth Generate the Diverse Palate Shapes of New World Leaf-Nosed Bats (Order Chiroptera, Family Phyllostomidae). Evol Biol 41, 12–21 (2014). https://doi.org/10.1007/s11692-013-9241-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-013-9241-8