Summary
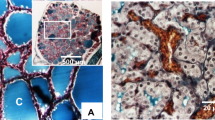
It is known that there is abnormal osteopontin (OPN) expression at the sites of atherosclerotic lesions. In the Apolipoprotein E gene knockout (ApoE-KO) mouse, a model of the atherosclerotic process, altered cholesterol metabolism with associated increase in OPN expression is evident at 12–22 weeks in the aorta and at 22 weeks in the heart. In this study, we analyzed another animal model of hypothyroid mice created by ingestion of propylthiouracil (PTU). After 2 weeks of PTU ingestion, the animals had significant decreases in thyroid hormones (T3 and T4) and immediate increases in blood lipids/cholesterol. Hypothyroid mice showed 1.3-, 1.5-, 2-fold increases in blood levels of total cholesterol, triglycerides, and low density lipoprotein-cholesterol respectively. Semi-quantitative RT-PCR analysis showed that hypothyroid mice had 1.4- to 2-fold increases of OPN mRNA expression in the aorta and 1.5-fold increases in the heart. Hypothyroid animals treated with T3 (5 μg/day for 6 days) or statin (0.2 mg/30 g for 2 weeks) reduce blood lipids and aortic OPN mRNA expression. Data obtained with ELISA analyses showed 1.5- and 1.7-fold increases in OPN protein in the aorta (10 weeks) and the heart (22 weeks), respectively. This increase is close to the mRNA expression in both tissues of hypothyroid mice. In addition, western blots showed several variants of OPN protein expressed in the aorta and the heart. The decrease in the 70 kDa OPN is accompanied by an increase in 45 kDa OPN in the aorta of hypothyroid mice. In contrast, only 45 kDa OPN is found in the heart of control and hypothyroid mice. These data indicate that the increase of OPN mRNA and protein expression occurs in cardiovascular tissues of hypothyroid mice.
Similar content being viewed by others
References
7. Butler WT (1989). The nature and significance of osteopontin. Connect Tissue Res 23:123–136
11. Denhardt D, Guo X (1993). Osteopontin: a protein with diverse functions. FASEB J 7:1475–1482
15. Franzen A, Heinegard D (1985). Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J 232:715–724
5. Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF, Senger DR (1992). Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell 3:1169–1180
40. Senger DR, Perruzzi CA, Papadopoulos A, Tenen DG (1989). Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta 1314:13–24
25. Kohri K, Nomura S, Kitamura Y, Nagata T, Yoshioka K, Iguchi M, Yamate T, Umekawa T, Suzuki Y, Sinohara H (1993). Structure and expression of the mRNA encoding urinary stone protein (osteopontin). J. Biol. Chem. 268:15180–15184
21. John GA, Burghardt RC, Spencer TE, Newton GR, Ott TL, Bazer FW (1999). Ovine osteopontin II Osteopontin and alpha(v) beta(3) integrin expression in the uterus and conceptus during the peri-implantation period. Biol Reprod 61: 892–899
12. Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS (2001). Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
17. Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM (1993). Osteopontin is elevated during neoinitima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest 92:1686–1696
32. O’Brien ER, Garvin MR, Stewart DK, Hinohara T, Simpson JB, Schwartz SM, Giachelli CM (1994). Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb 14:1648–1656
33. O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM (1995). Osteopontin is expressed in human aortic valvular lesions. Circulation 92:2163–2166
30. Murray CE, Giachelli CM, Schwartz SM, Vracko R (1994). Macrophages express osteopontin during repair of myocardial necrosis. Am J. Pathol 145:1450–1462
42. Singh K, Sirokman G, Communal C, Robinson KG, Conrad CH, Brooks WW, Bing OH, Colucci WS (1999). Myocardial osteopontin expression coincides with the development of heart failure. Hypertension 33:663–670
6. Burchfiel CM, Laws A, Benfante R, Goldberg RJ, Hwang LJ, Chiu D, Rodriguez BL, Curb JD, Sharp DS (1995). Combined effects of HDL cholesterol, triglyceride, and total cholesterol concentrations on 18-year risk of atherosclerotic disease. Circulation 92:1430–1436
28. Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T (2003). Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 23:1029–1034
31. Myers DL, Harmon KJ, Lindner V, Liaw L (2003). Alterations of arterial physiology in osteopontin-null mice. Arterioscler Thromb Vasc Biol 23:1021–1028
45. Ström Å, Franzén, Wängnerud C, Knutsson A-K, Heinegård D, Nilsson A-H (2004). Altered vascular remodeling in osteopontin-deficient atherosclerotic mice. J Vasc Res 41:314–322
14. Duntas LH (2002). Thyroid disease and lipids. Thyroid 12:287–293
39. Salter AM, Hayashi R, al-Seeni M, Brown NF, Bruce J, Sorensen O, Atkinson EA, Middleton B, Bleackley RC, Brindley DN (1991). Effects of hypothyroidism and high-fat feeding on mRNA concentrations for the low-density-lipoprotein receptor and on acyl-CoA:cholesterol acyltransferase activities in rat liver. Biochem J 276:825–832
43. Staels B, Van Tol A, Chan L, Will H, Verhoeven G, Auwerx J (1990) .Alterations in thyroid status modulate apolipoprotein, hepatic triglyceride lipase, and low density lipoprotein receptor in rats. Endocrinology 127:1144–1152
49. Thompson GR, Soutar AK, Spengel FA, Jadhav A, Gavigan SJ, Myant NB (1981). Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc Natl Acad Sci USA 78:2591–2595
8. Cappola AR, Ladenson PW (2003). Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab 88:2438–2444
24. Klein I, Ojamaa K (2001). Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509
2. Bakker O, Hudig F, Meijssen S, Wiersinga WM (1998). Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun 249:517–521
13. Diekman MJ, Anghelescu N, Endert E, Bakker O, Wiersinga WM (2000). Changes in plasma low-density lipoprotein (LDL)- and high-density lipoprotein cholesterol in hypo- and hyperthyroid patients are related to changes in free thyroxine, not to polymorphisms in LDL receptor or cholesterol ester transfer protein genes. J Clin Endocrinol Metab 85:1857–1862
46. Sundaram V, Hanna AN, Koneru L, Newman HA, Falko JM (1997). Both hypothyroidism and hyperthyroidism enhance low density lipoprotein oxidation. J Clin Endocrinol Metab 82:3421–3424
10. Danzi S, Klein I (2002). Thyroid hormone-regulated cardiac gene expression and cardiovascular disease. Thyroid 12:467–472
22. Johnson GA, Burghardt RC, Bazer FW, Spencer TE (2003). Osteopontin: roles in implantation and placentation. Biol Reprod 69:1458–1471
1. Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L (2001). Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J. Biol. Chem. 276: 28261–28267
3. Bautista DS, Denstedt J, Chambers AF, Harris JF (1996). Low-molecular-weight variants of osteopontin generated by serine proteinases in urine of patients with kidney stones. J Cell Biochem 61:402–409
35. Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N (1992). Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Proc Natl Acad Sci USA 89:4471–4475
36. Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL (1992). Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71:343–353
37. Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC (2002). Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation 105:2260–2265
38. Remaley AT, Schumacher UK, Amouzadeh HR, Brewer HB Jr, Hoeg JM (1995). Identification of novel differentially expressed hepatic genes in cholesterol-fed rabbits by a non-targeted gene approach. J Lipid Res 36:308–314
9. Chiba S, Okamoto H, Kon S, Kimura C, Murakami M, Inobe M, Matsui Y, Sugawara T, Shimizu T, Uede T, Kitabatake A (2002). Development of atherosclerosis in osteopontin transgenic mice. Heart Vessels 16:111–117
18. Giachelli CM, Schwartz SM, Liaw L (1995). Molecular and cellular biology of osteopontin role in cardiovascular disease. Trends Cardiovasc Med 5:88–95
20. Isoda K, Kamezawa Y, Ayaori M, Kusuhara M, Tada N, Ohsuzu F (2003). Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation 107:679–681
50. Towler DA,Bidder M, Latifi T, Coleman T, Semenkovich CF (1998). Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J. Biol. Chem. 273:30427–30434
26. Kawamura H, Yokote K, Asaumi S, Kobayashi K, Fujimoto M, Maezawa Y, Saito Y, Mori S (2004). High glucose-induced upregulation of osteopontin is mediated via Rho/Rho kinase pathway in cultured rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 276–281
47. Takemoto M, Yokote K, Nishimura M,Shigematsu T, Hasegawa T, Kon S, Uede T, Matsumoto T, Saito Y, Mori S (2000). Enhanced expression of osteopontin in human diabetic artery ad analysis of its functional role in accelerated atherogenesis. Arterioscler Thromb Vasc Biol 20:624–628
48. Takemoto M, Yokote K, Yamazaki M, Ridall AL, Butler WT, Matsumoto T, Tamura K, Saito Y, Mori S (1999). Enhanced expression of osteopontin by high glucose in cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun 258:722–726
4. Bidder M, Shao JS, Charlton-Kachigian N, Loewy AP, Semenkovich CF, and Towler DA (2002). Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J. Biol. Chem. 277:44485–44496
27. Liaw L, Lindner V, Schwaetz SM, Chambers AF, Giachelli CM (1995). Osteopontin and β3 integrin are coordinately expressed in regenerating endothelium in vivo and stimulate Arg-Gly-Asp-dependent endothelial migration in vitro. Circ Res 77:665–672
34. Patarca R, Saavedra RA, Cantor H (1993). Molecular and cellular basis of genetic resistance to bacterial infection: the role of the early T-lymphocyte activation-1/osteopontin gene. Crit Rev Immunol 13:225–246
29. Miyazaki Y, Setoguchi M, Yoshida S, Higuchi Y, Akizuki S, Yamamoto S (1990). The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J. Biol. Chem. 265:14432–14438
44. Stawowy P, Blaschke F, Pfautsch P, Goetze S, Lippek F, Wollert-Wulf B, Fleck E, Graf K (2002). Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur J Heart Fail 4:139–146
19. Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M (2000). Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20:2175–2183
16. Fresno M, McVay-Boudreau L, Nabel G, Cantor H (1981). Antigen-specific T lymphocyte clones. II. Purification and biological characterization of an antigen-specific suppressive protein synthesized by cloned T cells. J Exp Med 153:1260–1274
41. Senger DR, Perruzzi CA (1996). Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin. Biochim Biophys Acta 1314:13–24
23. Kaartinen MT, Pirhonen A, Linnala-Kankkunen A, Maenpaa PH (1999). Cross-linking of osteopontin by tissue transglutaminase increases its collagen binding properties. J. Biol. Chem. 274:1729–1735
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liou, Y., Chang, L., Liaw, J. et al. Osteopontin gene expression in the aorta and the heart of propylthiouracil-induced hypothyroid mice. J Biomed Sci 12, 869–880 (2005). https://doi.org/10.1007/s11373-005-9023-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11373-005-9023-0