Abstract
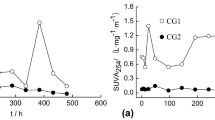
In former coal transformation plants (coking and gas ones), the major organic contamination of soils is coal tar, mainly composed of polycyclic aromatic compounds (PACs). Air oxidation of a fresh coal tar was chosen to simulate the abiotic natural attenuation impact on PAC-contaminated soils. Water-leaching experiments were subsequently performed on fresh and oxidized coal tars to study the influence of oxidation on dissolved organic matter (DOM) quality and quantity. The characterization of the DOM was performed using a combination of molecular and spectroscopic techniques (high-performance liquid chromatography–size-exclusion chromatography (HPLC-SEC), 3D fluorescence, and gas chromatography coupled with mass spectrometry (GC–MS)) and compared with the DOM from contaminated soils sampled on the field exposed to natural attenuation for several decades. An increase in the oxygenated polycyclic aromatic compound concentrations was observed with abiotic oxidation both in the coal tar and the associated DOM. Polycyclic aromatic hydrocarbon concentrations in the leachates exceeded pure water solubility limits, suggesting that co-solvation with other soluble organic compounds occurred. Furthermore, emission excitation matrix analysis combined with synchronous fluorescence spectra interpretation and size-exclusion chromatography suggests that oxidation induced condensation reactions which were responsible for the formation of higher-molecular weight compounds and potentially mobilized by water. Thus, the current composition of the DOM in aged soils may at least partly result from (1) a depletion in lower-molecular weight compounds of the initial contamination stock and (2) an oxidative condensation leading to the formation of a higher-molecular weight fraction. Abiotic oxidation and water leaching may therefore be a significant combination contributing to the evolution of coal tar-contaminated soils under natural attenuation.





Similar content being viewed by others
References
Benhabib K, Faure P, Sardin M, Simonnot MO (2010) Characteristics of a solid coal tar sampled from a contaminated soil and of the organics transferred into water. Fuel 89:352–359. doi:10.1016/j.fuel.2009.06.009
Biache C, Mansuy-Huault L, Faure P, Munier-Lamy C, Leyval C (2008) Effects of thermal desorption on the composition of two coking plant soils: impact on solvent extractable organic compounds and metal bioavailability. Environ Pollut 156:671–677. doi:10.1016/j.envpol.2008.06.020
Biache C, Ghislain T, Faure P, Mansuy-Huault L (2011) Low temperature oxidation of a coking plant soil organic matter and its major constituents: an experimental approach to simulate a long term evolution. J Hazard Mater 188:221–230. doi:10.1016/j.jhazmat.2011.01.102
Biache C, Faure P, Mansuy-Huault L, Cébron A, Beguiristain T, Leyval C (2013) Biodegradation of the organic matter in a coking plant soil and its main constituents. Org Geochem 56:10–18. doi:10.1016/j.orggeochem.2012.12.002
Birdwell JE, Engel AS (2010) Characterization of dissolved organic matter in cave and spring waters using UV–Vis absorbance and fluorescence spectroscopy. Org Geochem 41:270–280
Bispo A, Jourdain MJ, Jauzein M (1999) Toxicity and genotoxicity of industrial soils polluted by polycyclic aromatic hydrocarbons (PAHs). Org Geochem 30:947–952. doi:10.1016/s0146-6380(99)00078-9
Buczynska AJ, Geypens B, Van Grieken R, De Wael K (2013) Stable carbon isotopic ratio measurement of polycyclic aromatic hydrocarbons as a tool for source identification and apportionment—a review of analytical methodologies. Talanta 105:435–450. doi:10.1016/j.talanta.2012.10.075
Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Organic Geochemistry 37:1477–1488. doi:10.1016/j.oeggeochem.2006.06.022
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar Chem 51:325–346. doi:10.1016/0304-4203(95)00062-3
Coble PG (2007) Marine optical biogeochemistry: the chemistry of ocean color. Chem Rev 107:402–418. doi:10.1021/cr050350
Eom IC, Rast C, Veber AM, Vasseur P (2007) Ecotoxicity of a polycyclic aromatic hydrocarbon (PAH)-contaminated soil. Ecotoxicol Environ Saf 67:190–205
Faure P, Mansuy-Huault L, Su X (2007) Alkanes and hopanes for pollution source apportionment in coking plant soils. Environ Chem Lett 5:41–46. doi:10.1007/s10311-006-0066-x
Fernandez-Gomez WD, Quintana HAR, Daza CE, Lizcano FAR (2014) The effects of environmental aging on Colombian asphalts Fuel 115:321–328. doi:10.1016/j.fuel.2013.07.009
Ghislain T, Faure P, Biache C, Michels R (2010) Low-temperature, mineral-catalyzed air oxidation: a possible new pathway for PAH stabilization in sediments and soils. Environ Sci Technol 44:8547–8552. doi:10.1021/es102832r
Haeseler F, Blanchet D, Druelle V, Werner P, Vandecasteele J-P (1999) Analytical characterization of contaminated soils from former manufactured gas plants. Environ Sci Technol 33:825–830. doi:10.1021/es9805829
Huber SG, Wunderlich S, Scholer HF, Williams J (2010) Natural abiotic formation of furans in soil. Environ Sci Technol 44:5799–5804. doi:10.1021/es100704g
Huguet A, Vacher L, Relexans S, Saubusse S, Froidefond JM, Parlanti E (2009) Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org Geochem 40:706–719. doi:10.1016/j.orggeochem.2009.03.002
Huguet A et al (2010) New insights into the size distribution of fluorescent dissolved organic matter in estuarine waters. Org Geochem 41:595–610. doi:10.1016/j.orggeochem.2010.02.006
Hur J, Hwang SJ, Shin JK (2008) Using synchronous fluorescence technique as a water quality monitoring tool for an urban river. Water Air Soil Pollut 191:231–243. doi:10.1007/s11270-008-9620-4
Juhasz AL, Naidu R (2000) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzo a pyrene. Int Biodeterior Biodegrad 45:57–88. doi:10.1016/s0964-8305(00)00052-4
Keith L, Telliard W (1979) ES&T special report: priority pollutants: I—a perspective view. Environ Sci Technol 13:416–423. doi:10.1021/es60152a601
Kister J, Dou H (1986) Global characterization of the chemical constituents of coal by X scanner, UV fluorescence spectroscopy and FTIR spectroscopy. Fuel Process Technol 12:19–29. doi:10.1016/0378-3820(86)90064-0
Larionova AA, Zolotareva BN, Kolyagin YG, Kudeyarov VN (2013) Transformation of the organic matter in an agrogray soil and an agrochernozem in the course of the humification of corn biomass. Eurasian Soil Sci 46:854–861. doi:10.1134/s1064229313060045
Laurent F, Cebron A, Schwartz C, Leyval C (2012) Oxidation of a PAH polluted soil using modified Fenton reaction in unsaturated condition affects biological and physico-chemical properties. Chemosphere 86:659–664. doi:10.1016/j.chemosphere.2011.11.018
Lemaire J, Laurent F, Leyval C, Schwartz C, Bues M, Simonnot MO (2013) PAH oxidation in aged and spiked soils investigated by column experiments. Chemosphere 91:406–414. doi:10.1016/j.chemosphere.2012.12.003
Liao X, Ma D, Yan X, Yang L (2013) Distribution pattern of polycyclic aromatic hydrocarbons in particle-size fractions of coking plant soils from different depth. Environ Geochem Health 35:271–282. doi:10.1007/s10653-012-9482-y
Liu M et al (2010) Synthesis of metabolites of polycyclic aromatic hydrocarbons. Mini-Rev Org Chem 7:134–144
Luthy RG, Dzombak DA, Peters CA, Roy SB, Ramaswami A, Nakles DV, Nott BR (1994) Remediating tar-contaminated soils at manufactured-gas plant sites. Environ Sci Technol 28:A266–A276. doi:10.1021/es00055a002
Mackay D, Shiu WY (1977) Aqueous solubility of polynuclear aromatic hydrocarbons. J Chem Eng Data 22:399–402. doi:10.1021/je60075a012
Mahanty B, Pakshirajan K, Dasu VV (2011) Understanding the complexity and strategic evolution in PAH remediation research. Crit Rev Environ Sci Technol 41:1697–1746. doi:10.1080/10643389.2010.481586
Manzetti S (2013) Polycyclic aromatic hydrocarbons in the environment: environmental fate and transformation. Polycycl Aromat Compd 33:311–330. doi:10.1080/10406638.2013.781042
McCarthy JF, Wobber FJ (1993) Manipulation of groundwater colloids for environmental restoration. Lewis, Boca Raton
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr 46:38–48
Mendonca E, Picado A (2002) Ecotoxicological monitoring of remediation in a coke oven soil. Environ Toxicol 17:74–79. doi:10.1002/tox.10034
Mille G, Guiliano M, Kister J (1988) Analysis and evolution of coals: UV fluorescence spectroscopy study (demineralized coals-oxidized coals). Org Geochem 13:947–952. doi:10.1016/0146-6380(88)90276-8
Morillo E et al (2007) Soil pollution by PAHs in urban soils: a comparison of three European cities. J Environ Monit 9:1001. doi:10.1039/b705955h
Northcott GL, Jones KC (2001) Partitioning, extractability, and formation of nonextractable PAH residues in soil. 1. Compound differences in aging and sequestration. Environ Sci Technol 35:1103–1110. doi:10.1021/es000071y
Parlanti E, Worz K, Geoffroy L, Lamotte M (2000) Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org Geochem 31:1765–1781. doi:10.1016/s0146-6380(00)00124-8
Paul-Dauphin S, Karaca F, Morgan TJ, Millan-Agorio M, Herod AA, Kandiyoti R (2007) Probing size exclusion mechanisms of complex hydrocarbon mixtures: the effect of altering eluent compositions. Energy Fuel 21:3484–3489. doi:10.1021/ef700410e
Pernot A, Ouvrard S, Leglize P, Faure P (2013) Protective role of fine silts for PAH in a former industrial soil. Environ Pollut 179:81–87. doi:10.1016/j.envpol.2013.03.068
Reichenberg F, Karlson UG, Gustafsson O, Long SM, Pritchard PH, Mayer P (2010) Low accessibility and chemical activity of PAHs restrict bioremediation and risk of exposure in a manufactured gas plant soil. Environ Pollut 158:1214–1220. doi:10.1016/j.envpol.2010.01.031
Rollin C, Quiot F (2005) Hydrocarbures aromatiques polycycliques-Final repport n°66244-DESP-R01. INERIS, Aix-en-Provence
Sánchez-Trujillo MA, Morillo E, Villaverde J, Lacorte S (2013) Comparative effects of several cyclodextrins on the extraction of PAHs from an aged contaminated soil. Environ Pollut 178:52–58. doi:10.1016/j.envpol.2013.02.029
Sayara T, Borràs E, Caminal G, Sarrà M, Sánchez A (2011) Bioremediation of PAHs-contaminated soil through composting: influence of bioaugmentation and biostimulation on contaminant biodegradation. Int Biodeterior Biodegrad 65:859–865. doi:10.1016/j.ibiod.2011.05.006
Schwartz CF, Florentin L, Charpentier D, Muzika S, Morel J-L (2001) Le pédologue en milieux industriels et urbains. Etude de Gestion des Sols 8:135–148
Senturk Z (2013) Analysis of carcinogenic polycyclic aromatic hydrocarbons (PAHs): an overview of modern electroanalytical techniques and their applications. Curr Drug Deliv 10:76–91
Shuterland JB, Raffi F, Khan AA, Cerniglia CE (1995) Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young LY, Cerniglia CE (eds) Microbial transformation and degradation of toxic organic chemicals. Wiley, New York, pp 296–306
Stedmon CA, Markager S (2005) Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol Oceanogr 50:686–697
Teoh WH, Mammucari R, de Melo S, Foster NR (2013) Solubility and solubility modeling of polycyclic aromatic hydrocarbons in subcritical water. Ind Eng Chem Res 52:5806–5814. doi:10.1021/ie302124e
Tiruta-Barna L, Mahjoub B, Faure L, Hanna K, Bayard R, Gourdon R (2006) Assessment of the multi-compound non-equilibrium dissolution behaviour of a coal tar containing PAHs and phenols into water. J Hazard Mater 132:277–286. doi:10.1016/j.jhazmat.2005.10.013
Tsibart AS, Gennadiev AN (2013) Polycyclic aromatic hydrocarbons in soils: sources, behavior, and indication significance (a review). Eurasian Soil Sci 46:728–741
USEPA (1999) Use of monitored natural attenuation at superfund, RCRA corrective action, and underground storage tank sites vol OSWER Directive number 9200.4-17P. Office of Solid Waste and Emergency Response, Washington, DC
USEPA (2008) Coke production—final report. Emission factor documentation for AP-42 section 12.2. USEPA, Office of Air Quality Planning and Standards, Research Triangle Park
Vulava VM, McKay LD, Driese SG, Menn F-M, Sayler GS (2007) Distribution and transport of coal tar-derived PAHs in fine-grained residuum. Chemosphere 68:554–563. doi:10.1016/j.chemosphere.2006.12.086
Vulava VM, McKay LD, Broholm MM, McCarthy JF, Driese SG, Sayler GS (2012) Dissolution and transport of coal tar compounds in fractured clay-rich residuum. J Hazard Mater 203:283–289. doi:10.1016/j.jhazmat.2011.12.023
Wang C, Guo WD, Guo ZR, Wei J, Zhang B, Ma ZY (2013) Characterization of dissolved organic matter in groundwater from the coastal Dagu River watershed, china using fluorescence excitation-emission matrix spectroscopy. Spectrosc Spectr Anal 33:2460–2465. doi:10.3964/j.issn.1000-0593(2013)09-2460-06
Wang K, Chen XX, Zhu ZQ, Huang HG, Li TQ, Yang XE (2014) Dissipation of available benzo a pyrene in aging soil co-contaminated with cadmium and pyrene. Environ Sci Pollut Res 21:962–971. doi:10.1007/s11356-013-1960-y
Wilcke W (2000) Polycyclic aromatic hydrocarbons (PAHs) in soil—a review. J Plant Nutr Soil Sci Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 163:229–248. doi:10.1002/1522-2624(200006)163:3<229::aid-jpln229>3.0.co;2-6
Yi H, Crowley DE (2007) Biostimulation of PAH degradation with plants containing high concentrations of linoleic acid. Environ Sci Technol 41:4382–4388. doi:10.1021/es062397y
Yu H, Song Y, Tu X, Du E, Liu R, Peng J (2013) Assessing removal efficiency of dissolved organic matter in wastewater treatment using fluorescence excitation emission matrices with parallel factor analysis and second derivative synchronous fluorescence. Bioresour Technol 144:595–601. doi:10.1016/j.biortech.2013.07.025
Zhao Z-B, Liu K, Xie W, Pan W-P, Riley JT (2000) Soluble polycyclic aromatic hydrocarbons in raw coals. J Hazard Mater 73:77–85. doi:10.1016/S0304-3894(99)00178-8
Zhou BX, Cai WM, Zou LZ (2003) Thermodynamic functions for transfer of anthracene from water to (water plus alcohol) mixtures at 298.15 K. J Chem Eng Data 48:742–745
Zsolnay A, Baigar E, Jimenez M, Steinweg B, Saccomandi F (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38:45–50. doi:10.1016/s0045-6535(98)00166-0
Acknowledgments
The authors thank the French Ministère de l’Enseignement Supérieur et de la Recherche for the financial support given to this study. They also thank the Groupement d’Intérêt Scientifique pour les Friches Industrielles (GISFI) for providing the coking plant soil samples. Finally, they thank J. Rochester for her welcomed review of the quality of the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hanser, O., Biache, C., Boulangé, M. et al. Evolution of dissolved organic matter during abiotic oxidation of coal tar—comparison with contaminated soils under natural attenuation. Environ Sci Pollut Res 22, 1431–1443 (2015). https://doi.org/10.1007/s11356-014-3465-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3465-8